TFR Cells Express Functional CCR6 But It Is Dispensable for Their Development and Localization During Splenic Humoral Immune Responses
- PMID: 35812408
- PMCID: PMC9257258
- DOI: 10.3389/fimmu.2022.873586
TFR Cells Express Functional CCR6 But It Is Dispensable for Their Development and Localization During Splenic Humoral Immune Responses
Abstract
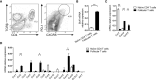
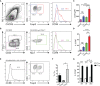
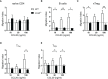
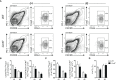
Follicular T cells including T follicular helper (TFH) and T follicular regulatory (TFR) cells are essential in supporting and regulating the quality of antibody responses that develop in the germinal centre (GC). Follicular T cell migration during the propagation of antibody responses is largely attributed to the chemokine receptor CXCR5, however CXCR5 is reportedly redundant in migratory events prior to formation of the GC, and CXCR5-deficient TFH and TFR cells are still capable of localizing to GCs. Here we comprehensively assess chemokine receptor expression by follicular T cells during a model humoral immune response in the spleen. In addition to the known follicular T cell chemokine receptors Cxcr5 and Cxcr4, we show that follicular T cells express high levels of Ccr6, Ccr2 and Cxcr3 transcripts and we identify functional expression of CCR6 protein by both TFH and TFR cells. Notably, a greater proportion of TFR cells expressed CCR6 compared to TFH cells and gating on CCR6+CXCR5hiPD-1hi T cells strongly enriched for TFR cells. Examination of Ccr6-/- mice revealed that CCR6 is not essential for development of the GC response in the spleen, and mixed bone marrow chimera experiments found no evidence for an intrinsic requirement for CCR6 in TFR cell development or localisation during splenic humoral responses. These findings point towards multiple functionally redundant chemotactic signals regulating T cell localisation in the GC.
Keywords: CCR6; T follicular helper (Tfh) cell; T follicular regulatory (Tfr) cells; chemokine receptor; germinal center T cell.
Copyright © 2022 Bastow, Kara, Tyllis, Vinuesa, McColl and Comerford.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

