Aqueous Extract of Descuraniae Semen Attenuates Lipopolysaccharide-Induced Inflammation and Apoptosis by Regulating the Proteasomal Degradation and IRE1α-Dependent Unfolded Protein Response in A549 Cells
- PMID: 35812413
- PMCID: PMC9265213
- DOI: 10.3389/fimmu.2022.916102
Aqueous Extract of Descuraniae Semen Attenuates Lipopolysaccharide-Induced Inflammation and Apoptosis by Regulating the Proteasomal Degradation and IRE1α-Dependent Unfolded Protein Response in A549 Cells
Abstract
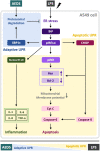
Background: Lipopolysaccharide (LPS)-induced acute lung injury (ALI) induces endoplasmic reticulum stress, unfolded protein response (UPR), apoptosis, and inflammation. Inositol-requiring enzyme 1 (IRE1)-α is important for adaptive and apoptotic UPR determination during ER stress. The aqueous extract of Descuraniae Semen (AEDS) is reported to be a safe and effective herb for the treatment of pulmonary edema as it shows anti-inflammatory activities.
Methods: We investigated the effects of AEDS on LPS-induced ALI in A549 cells with respect to the regulation of IRE1α-dependent UPR, proteasomal degradation, mitochondrial membrane potential (MtMP), inflammation, and apoptosis.
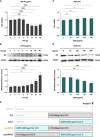
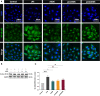
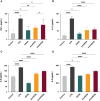
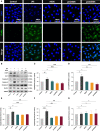
Results: AEDS attenuated ER stress by regulating the proteasomal degradation. LPS induced ER stress [binding immunoglobulin protein (BiP), phosphorylated IRE1α, sliced X-box binding protein 1 [XBP1s], phosphorylated cJUN NH2-terminal kinase (pJNK), B-cell lymphoma (Bcl)-2-associated X (Bax), Bcl-2], inflammation (nucleus factor-kappa B (NF-κB) p65 nuclear translocation, nucleus NF-κB, pro-inflammatory cytokines] and apoptosis [C/EBP homologous protein (CHOP), cytochrome c, caspase-8, and caspase-6, and TUNEL] were significantly attenuated by AEDS treatment in A549 cells. AEDS prevents LPS-induced decreased expression of MtMP in A549 cells.
Conclusions: AEDS attenuated LPS-induced inflammation and apoptosis by regulating proteasomal degradation, promoting IRE1α-dependent adaptive UPR, and inhibiting IRE1α-dependent apoptotic UPR. Moreover, IRE1α-dependent UPR plays a pivotal role in the mechanisms of LPS-induced ALI. Based on these findings, AEDS is suggested as a potential therapeutic option for treating patients with ALI.
Keywords: Descuraniae semen; IRE1α; acute lung injury; apoptosis; inflammation; lipopolysaccharide; unfolded protein response.
Copyright © 2022 Hsieh, Peng, Liu, Kuo, Tzeng, Wang, Lan and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Aqueous extract of Descuraniae Semen attenuates lipopolysaccharide-induced inflammation by inhibiting ER stress and WNK4-SPAK-NKCC1 pathway.J Cell Mol Med. 2024 Aug;28(15):e18589. doi: 10.1111/jcmm.18589. J Cell Mol Med. 2024. PMID: 39135202 Free PMC article.
-
Genipin attenuates mitochondrial-dependent apoptosis, endoplasmic reticulum stress, and inflammation via the PI3K/AKT pathway in acute lung injury.Int Immunopharmacol. 2019 Nov;76:105842. doi: 10.1016/j.intimp.2019.105842. Epub 2019 Aug 26. Int Immunopharmacol. 2019. PMID: 31466050
-
Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1α/NF-κB signaling pathway.Food Funct. 2018 Apr 25;9(4):2386-2397. doi: 10.1039/c7fo01406f. Food Funct. 2018. PMID: 29594285
-
Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response.Free Radic Biol Med. 2013 Dec;65:162-174. doi: 10.1016/j.freeradbiomed.2013.06.020. Epub 2013 Jun 19. Free Radic Biol Med. 2013. PMID: 23792277 Review.
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
Cited by
-
Jing Si Herbal Tea Modulates Macrophage Polarization and Inflammatory Signaling in LPS-Induced Inflammation.Int J Med Sci. 2024 Nov 11;21(15):3046-3057. doi: 10.7150/ijms.100720. eCollection 2024. Int J Med Sci. 2024. PMID: 39628684 Free PMC article.
-
Signaling pathways and potential therapeutic targets in acute respiratory distress syndrome (ARDS).Respir Res. 2024 Jan 13;25(1):30. doi: 10.1186/s12931-024-02678-5. Respir Res. 2024. PMID: 38218783 Free PMC article. Review.
-
Developments in the connection between epithelial‑mesenchymal transition and endoplasmic reticulum stress (Review).Int J Mol Med. 2025 Jul;56(1):102. doi: 10.3892/ijmm.2025.5543. Epub 2025 May 9. Int J Mol Med. 2025. PMID: 40341397 Free PMC article. Review.
-
Antioxidant, Antimicrobial, and Anti-Inflammatory Effects of Liriodendron chinense Leaves.ACS Omega. 2024 Jun 10;9(25):27002-27016. doi: 10.1021/acsomega.3c10269. eCollection 2024 Jun 25. ACS Omega. 2024. PMID: 38947843 Free PMC article.
-
Aqueous extract of Descuraniae Semen attenuates lipopolysaccharide-induced inflammation by inhibiting ER stress and WNK4-SPAK-NKCC1 pathway.J Cell Mol Med. 2024 Aug;28(15):e18589. doi: 10.1111/jcmm.18589. J Cell Mol Med. 2024. PMID: 39135202 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

