Enhanced Therapeutic Efficacy of Combining Losartan and Chemo-Immunotherapy for Triple Negative Breast Cancer
- PMID: 35812418
- PMCID: PMC9259940
- DOI: 10.3389/fimmu.2022.938439
Enhanced Therapeutic Efficacy of Combining Losartan and Chemo-Immunotherapy for Triple Negative Breast Cancer
Abstract
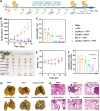
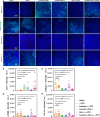
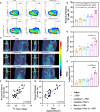
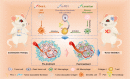
Triple-negative breast cancer (TNBC) is a particularly aggressive subtype of breast cancer, which is relatively resistant to anti-programmed cell death-1 (α-PD1) therapy, characterized as non-immunogenic, dense stroma and accumulation of M2 tumor-associated macrophages (TAMs). Despite progress in strategies to deplete extracellular matrix (ECM) and enhance tumor-cell immunogenicity, the combinatorial anti-cancer effects with α-PD1 need to be explored. Here, we applied doxorubicin hydrochloride liposome (Dox-L) as immunogenic cell death (ICD)-inducing nano-chemotherapy and used losartan as stroma-depleting agent to improve α-PD1 efficacy (Losartan + Dox-L + α-PD1). The results showed that losartan could cause ECM reduction, facilitating enhanced delivery of Dox-L and further dendritic cell (DC) maturation. Additionally, losartan could also alleviate hypoxia for TNBC, thus reprogramming pro-cancer M2 TAMs to anti-cancer M1 TAMs, successfully overcoming immune-suppressive microenvironment. These modifications led to a significant increase in T cells' infiltration and augmented anti-tumor immunity as exemplified by the notable reduction in tumor size and lung metastases. In summary, our findings support that combined treatment of losartan with Dox-L normalizes immunological-cold microenvironment, improves immuno-stimulation and optimizes the efficacy of TNBC immunotherapy. A novel combinational strategy with FDA-approved compounds proposed by the study may potentially be useful in TNBC clinical treatment.
Keywords: chemotherapy; extracellular matrix; immunotherapy; positron emission tomography (PET); triple-negative breast cancer.
Copyright © 2022 Zhao, He, Qin, Liu, Jiang, Wang, Wu, Zhou, Yu, Liu, Zhang and Tian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Celastrol-loaded ginsenoside Rg3 liposomes enhance anti-programmed death ligand 1 immunotherapy by inducing immunogenic cell death in triple-negative breast cancer.Phytomedicine. 2025 Apr;139:156514. doi: 10.1016/j.phymed.2025.156514. Epub 2025 Feb 16. Phytomedicine. 2025. PMID: 39986227
-
Promotion of triple negative breast cancer immunotherapy by combining bioactive radicals with immune checkpoint blockade.Acta Biomater. 2025 Mar 1;194:305-322. doi: 10.1016/j.actbio.2025.01.015. Epub 2025 Jan 11. Acta Biomater. 2025. PMID: 39805523
-
Enhancing TNBC Chemo-immunotherapy via combination reprogramming tumor immune microenvironment with Immunogenic Cell Death.Int J Pharm. 2021 Apr 1;598:120333. doi: 10.1016/j.ijpharm.2021.120333. Epub 2021 Feb 1. Int J Pharm. 2021. PMID: 33540008
-
Targeting immune cells in tumor microenvironment in triple negative breast cancer therapy: future perspective to overcome doxorubicin resistance and toxicity.Med Oncol. 2025 Apr 4;42(5):150. doi: 10.1007/s12032-025-02712-6. Med Oncol. 2025. PMID: 40183881 Review.
-
Progress and pitfalls in the use of immunotherapy for patients with triple negative breast cancer.Expert Opin Investig Drugs. 2022 Jun;31(6):567-591. doi: 10.1080/13543784.2022.2049232. Epub 2022 Mar 9. Expert Opin Investig Drugs. 2022. PMID: 35240902 Review.
Cited by
-
Several first-line anti-hypertensives act on fibrosarcoma progression and PD1ab blockade therapy.J Orthop Surg Res. 2024 Feb 19;19(1):147. doi: 10.1186/s13018-024-04627-w. J Orthop Surg Res. 2024. PMID: 38373964 Free PMC article.
-
Distinct mRNA expression profiles and miRNA regulators of the PI3K/AKT/mTOR pathway in breast cancer: insights into tumor progression and therapeutic targets.Front Oncol. 2025 Jan 9;14:1515387. doi: 10.3389/fonc.2024.1515387. eCollection 2024. Front Oncol. 2025. PMID: 39850811 Free PMC article.
-
Collagen Remodeling along Cancer Progression Providing a Novel Opportunity for Cancer Diagnosis and Treatment.Int J Mol Sci. 2022 Sep 10;23(18):10509. doi: 10.3390/ijms231810509. Int J Mol Sci. 2022. PMID: 36142424 Free PMC article. Review.
-
In Vitro Drug Repurposing: Focus on Vasodilators.Cells. 2023 Feb 20;12(4):671. doi: 10.3390/cells12040671. Cells. 2023. PMID: 36831338 Free PMC article. Review.
-
Engineering the glioblastoma microenvironment with bioactive nanoparticles for effective immunotherapy.RSC Adv. 2023 Oct 27;13(45):31411-31425. doi: 10.1039/d3ra01153d. eCollection 2023 Oct 26. RSC Adv. 2023. PMID: 37901257 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

