Fibrin(ogen) Is Constitutively Expressed by Differentiated Intestinal Epithelial Cells and Mediates Wound Healing
- PMID: 35812445
- PMCID: PMC9258339
- DOI: 10.3389/fimmu.2022.916187
Fibrin(ogen) Is Constitutively Expressed by Differentiated Intestinal Epithelial Cells and Mediates Wound Healing
Abstract
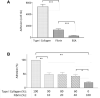
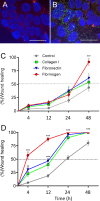
Fibrinogen is a large molecule synthesized in the liver and released in the blood. Circulating levels of fibrinogen are upregulated after bleeding or clotting events and support wound healing. In the context of an injury, thrombin activation drives conversion of fibrinogen to fibrin. Fibrin deposition contains tissue damage, stops blood loss, and prevents microbial infection. In most circumstances, fibrin needs to be removed to allow the resolution of inflammation and tissue repair, whereas failure of this may lead to the development of various disorders. However, the contribution of fibrinogen to tissue inflammation and repair is likely to be context-dependent. In this study, the concept that fibrin needs to be removed to allow tissue repair and to reduce inflammation is challenged by our observations that, in the intestine, fibrinogen is constitutively produced by a subset of intestinal epithelial cells and deposited at the basement membrane as fibrin where it serves as a substrate for wound healing under physiological conditions such as epithelial shedding at the tip of the small intestinal villus and surface epithelium of the colon as well as under pathological conditions that require rapid epithelial repair. The functional integrity of the intestine is ensured by the constant renewal of its simple epithelium. Superficial denuding of the epithelial cell layer occurs regularly and is rapidly corrected by a process called restitution that can be influenced by various soluble and insoluble factors. Epithelial cell interaction with the extracellular matrix greatly influences the healing process by acting on cell morphology, adhesion, and migration. The functional contribution of a fibrin(ogen) matrix in the intestine was studied under physiological and pathological contexts. Our results (immunofluorescence, immunoelectron microscopy, and quantitative PCR) show that fibrin(ogen) is a novel component of the basement membrane associated with the differentiated epithelial cell population in both the small intestine and colon. Fibrin(ogen) alone is a weak ligand for epithelial cells and behaves as an anti-adhesive molecule in the presence of type I collagen. Furthermore, the presence of fibrin(ogen) significantly shortens the time required to achieve closure of wounded epithelial cell monolayers and co-cultures in a PI3K-dependent manner. In human specimens with Crohn's disease, we observed a major accumulation of fibrin(ogen) throughout the tissue and at denuded sites. In mice in which fibrin formation was inhibited with dabigatran treatment, dextran sulfate sodium administration provoked a significant increase in the disease activity index and pathological features such as mucosal ulceration and crypt abscess formation. Taken together, these results suggest that fibrin(ogen) contributes to epithelial healing under both normal and pathological conditions.
Keywords: Crohn’s disease; PI3K; dabigatran; epithelial restitution; fibrinogen; human; intestinal homeostasis; mouse.
Copyright © 2022 Seltana, Cloutier, Reyes Nicolas, Khalfaoui, Teller, Perreault and Beaulieu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Fallah S, Sénicourt B, Beaulieu J-F. Proliferation in the Gastrointestinal Epithelium. In: Kuipers EJ, editor. Encyclopedia of Gastroenterology (Second Edition). Oxford: Academic Press; (2020). p. 304–10.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

