Antibiofilm properties of Clitoria ternatea flower anthocyanin-rich fraction towards Pseudomonas aeruginosa
- PMID: 35812712
- PMCID: PMC9260092
- DOI: 10.1099/acmi.0.000343
Antibiofilm properties of Clitoria ternatea flower anthocyanin-rich fraction towards Pseudomonas aeruginosa
Abstract
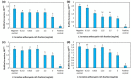
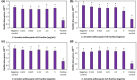
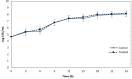
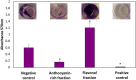
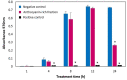
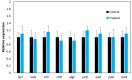
In Asia, Clitoria ternatea flowers are commonly used as a traditional medicinal herb and as a food colourant. Their bioactive compounds have anti-inflammatory, anti-microbial and anti-biofilm activities. Pseudomonas aeruginosa is one of the major pathogens that cause biofilm-associated infections resulting in an increase in antimicrobial resistance. Hence, the aim of this study was to investigate if the anti-biofilm properties of the anthocyanin-rich fraction of C. ternatea flowers were effective against P. aeruginosa . The effect of the anthocyanin-rich fraction of C. ternatea flowers on P. aeruginosa biofilms formed on a polystyrene surface was determined using the crystal violet assay and scanning electron microscopy (SEM). The anthocyanin-rich fraction reduced biofilm formation by four P. aeruginosa strains with a minimum biofilm inhibitory concentration value ranging between 0.625 and 5.0 mg ml-1. We further show that the biofilm-inhibiting activity of C. ternatea flowers is not due to the flavonols but is instead attributed to the anthocyanins, which had significant biofilm inhibitory activity (64.0±1.1 %) at 24 h in a time-response study. The anthocyanin-rich fraction also significantly reduced bacterial attachment on the polystyrene by 1.1 log c.f.u. cm-2 surface based on SEM analysis. Hence, anthocyanins from C. ternatea flowers have potential as an agent to decrease the risk of biofilm-associated infections.
Keywords: anthocyanin; antimicrobial; biofilm-inhibiting activity; blue pea; crystal violet; flavonol.
© 2022 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Antibiofilm activity of Clitoria ternatea flowers anthocyanin fraction against biofilm-forming oral bacteria.FEMS Microbiol Lett. 2025 Jan 10;372:fnaf035. doi: 10.1093/femsle/fnaf035. FEMS Microbiol Lett. 2025. PMID: 40128011 Free PMC article.
-
Antioxidant, cytotoxic, and antibacterial activities of Clitoria ternatea flower extracts and anthocyanin-rich fraction.Sci Rep. 2022 Sep 1;12(1):14890. doi: 10.1038/s41598-022-19146-z. Sci Rep. 2022. PMID: 36050436 Free PMC article.
-
Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals.J Food Sci Technol. 2021 Jun;58(6):2054-2067. doi: 10.1007/s13197-020-04745-3. Epub 2020 Sep 1. J Food Sci Technol. 2021. PMID: 33967304 Free PMC article. Review.
-
Anthocyanins From Clitoria ternatea Flower: Biosynthesis, Extraction, Stability, Antioxidant Activity, and Applications.Front Plant Sci. 2021 Dec 17;12:792303. doi: 10.3389/fpls.2021.792303. eCollection 2021. Front Plant Sci. 2021. PMID: 34975979 Free PMC article. Review.
-
Metabolic perturbations and key pathways associated with the bacteriostatic activity of Clitoria ternatea flower anthocyanin fraction against Escherichia coli.Access Microbiol. 2023 Jun 28;5(6):acmi000535.v5. doi: 10.1099/acmi.0.000535.v5. eCollection 2023. Access Microbiol. 2023. PMID: 37424541 Free PMC article.
Cited by
-
Antibiofilm activity of Clitoria ternatea flowers anthocyanin fraction against biofilm-forming oral bacteria.FEMS Microbiol Lett. 2025 Jan 10;372:fnaf035. doi: 10.1093/femsle/fnaf035. FEMS Microbiol Lett. 2025. PMID: 40128011 Free PMC article.
-
Biofilm destruction activity of α-tocopherol against Staphylococcus aureus, Proteus mirabilis, and Pseudomonas aeruginosa.FEMS Microbiol Lett. 2025 Jan 10;372:fnaf020. doi: 10.1093/femsle/fnaf020. FEMS Microbiol Lett. 2025. PMID: 39904546 Free PMC article.
-
Topical antifungal keratitis therapeutic potential of Clitoria ternatea Linn. flower extract: phytochemical profiling, in silico modelling, and in vitro biological activity assessment.Front Microbiol. 2024 Jan 24;15:1343988. doi: 10.3389/fmicb.2024.1343988. eCollection 2024. Front Microbiol. 2024. PMID: 38328419 Free PMC article. Review.
-
Supernatant of plant-associated bacteria potency against biofilms formed by foodborne pathogen and food spoilage bacteria.BMC Res Notes. 2024 Nov 14;17(1):338. doi: 10.1186/s13104-024-06997-0. BMC Res Notes. 2024. PMID: 39543762 Free PMC article.
-
Antioxidant, cytotoxic, and antibacterial activities of Clitoria ternatea flower extracts and anthocyanin-rich fraction.Sci Rep. 2022 Sep 1;12(1):14890. doi: 10.1038/s41598-022-19146-z. Sci Rep. 2022. PMID: 36050436 Free PMC article.
References
-
- Kirchhelle C. Pharming animals: a global history of antibiotics in food production (1935–2017) Palgrave Commun. 2018;4:1–13. doi: 10.1057/s41599-018-0152-2. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
