Treatment Effects of Intra-Articular Allogenic Mesenchymal Stem Cell Secretome in an Equine Model of Joint Inflammation
- PMID: 35812845
- PMCID: PMC9257274
- DOI: 10.3389/fvets.2022.907616
Treatment Effects of Intra-Articular Allogenic Mesenchymal Stem Cell Secretome in an Equine Model of Joint Inflammation
Abstract
Background: Allogenic mesenchymal stem cell (MSC) secretome is a novel intra-articular therapeutic that has shown promise in in vitro and small animal models and warrants further investigation.
Objectives: To investigate if intra-articular allogenic MSC-secretome has anti-inflammatory effects using an equine model of joint inflammation.
Study design: Randomized positively and negatively controlled experimental study.
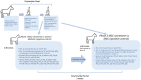
Method: In phase 1, joint inflammation was induced bilaterally in radiocarpal joints of eight horses by injecting 0.25 ng lipopolysaccharide (LPS). After 2 h, the secretome of INFy and TNFα stimulated allogeneic equine MSCs was injected in one randomly assigned joint, while the contralateral joint was injected with medium (negative control). Clinical parameters (composite welfare scores, joint effusion, joint circumference) were recorded, and synovial fluid samples were analyzed for biomarkers (total protein, WBCC; eicosanoid mediators, CCL2; TNFα; MMP; GAGs; C2C; CPII) at fixed post-injection hours (PIH 0, 8, 24, 72, and 168 h). The effects of time and treatment on clinical and synovial fluid parameters and the presence of time-treatment interactions were evaluated. For phase 2, allogeneic MSC-secretome vs. allogeneic equine MSCs (positive control) was tested using a similar methodology.
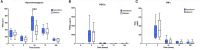
Results: In phase 1, the joint circumference was significantly (p < 0.05) lower in the MSC-secretome treated group compared to the medium control group at PIH 24, and significantly higher peak synovial GAG values were noted at PIH 24 (p < 0.001). In phase 2, no significant differences were noted between the treatment effects of MSC-secretome and MSCs.
Main limitations: This study is a controlled experimental study and therefore cannot fully reflect natural joint disease. In phase 2, two therapeutics are directly compared and there is no negative control.
Conclusions: In this model of joint inflammation, intra-articular MSC-secretome injection had some clinical anti-inflammatory effects. An effect on cartilage metabolism, evident as a rise in GAG levels was also noted, although it is unclear whether this could be considered a beneficial or detrimental effect. When directly comparing MSC-secretome to MSCs in this model results were comparable, indicating that MSC-secretome could be a viable off-the-shelf alternative to MSC treatment.
Keywords: equine model; joint inflammation; lipopolysaccharide (LPS); mesenchymal stem cells; secretome.
Copyright © 2022 Kearney, Khatab, van Buul, Plomp, Korthagen, Labberté, Goodrich, Kisiday, Van Weeren, van Osch and Brama.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Colbath AC, Dow SW, Hopkins LS, Phillips JN, McIlwraith CW, Goodrich LR. Single and repeated intra-articular injections in the tarsocrural joint with allogeneic and autologous equine bone marrow-derived mesenchymal stem cells are safe, but did not reduce acute inflammation in an experimental interleukin-1beta model of synovitis. Equine Vet J. (2019) 52:601–12. 10.1111/evj.13222 - DOI - PMC - PubMed
-
- EMA . First Stem Cell-Based Veterinary Medicine Recommended for Marketing Authorisation. Available online at: https://www.ema.europa.eu/en/documents/press-release/first-stem-cell-bas... (accessed May 25, 2021).
LinkOut - more resources
Full Text Sources

