Identification of Pulmonary Infections With Porcine Rotavirus A in Pigs With Respiratory Disease
- PMID: 35812854
- PMCID: PMC9260157
- DOI: 10.3389/fvets.2022.918736
Identification of Pulmonary Infections With Porcine Rotavirus A in Pigs With Respiratory Disease
Abstract
While rotavirus (RV) is primarily known to cause gastroenteritis in many animals, several epidemiological studies have shown concurrent respiratory symptoms with fecal and nasal virus shedding. However, respiratory RV infections have rarely been investigated. By screening clinical samples submitted for diagnostic testing, porcine rotavirus A (RVA) was detected by quantitative reverse transcription PCR (qRT-PCR) in 28 out of 91 (30.8%) lungs obtained from conventionally reared pigs with respiratory signs. Among the positive cases, intensive RVA signals were mainly localized in alveolar macrophages (n = 3) and bronchiolar epithelial cells (n = 1) by RNAscope® in situ hybridization (ISH). The signals of RVA in bronchiolar epithelial cells were verified by ISH with different probes, immunohistochemistry, and transmission electron microscopy. Furthermore, additional cases with RVA ISH-positive signals in alveolar macrophages (n = 9) and bronchial epithelial cells (n = 1) were identified by screening 120 archived formalin-fixed and paraffin-embedded lung samples using tissue microarrays. Overall, our study showed a high frequency of RVA detection in lungs from conventional pigs with respiratory disease. Further research is needed to determine if RVA infection in the respiratory epithelium correlates with nasal shedding of rotavirus and its contribution to respiratory disease.
Keywords: enteritis; extraintestinal rotavirus; interstitial pneumonia; porcine respiratory disease complex; rotavirus A.
Copyright © 2022 Nelsen, Lager, Stasko, Nelson, Lin and Hause.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
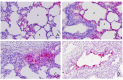

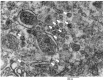
Comment in
-
Commentary: Identification of pulmonary infections with porcine Rotavirus A in pigs with respiratory disease.Front Vet Sci. 2023 Jan 17;10:1102602. doi: 10.3389/fvets.2023.1102602. eCollection 2023. Front Vet Sci. 2023. PMID: 36733638 Free PMC article. No abstract available.
References
-
- International Committee on Taxonomy of Viruses. (2011). Reoviridae. Available online at: https://talk.ictvonline.org/ictv-reports/ictv_9th_report/dsrna-viruses-2... (accessed February 2, 2022.).
LinkOut - more resources
Full Text Sources

