Secretion of IFN-γ by Transgenic Mammary Epithelial Cells in vitro Reduced Mastitis Infection Risk in Goats
- PMID: 35812858
- PMCID: PMC9263845
- DOI: 10.3389/fvets.2022.898635
Secretion of IFN-γ by Transgenic Mammary Epithelial Cells in vitro Reduced Mastitis Infection Risk in Goats
Abstract
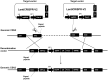
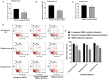
Mastitis results in great economic loss to the dairy goat industry. Many approaches have attempted to decrease the morbidity associated with this disease, and among these, transgenic strategy have been recognized as a potential approach. A previous mammalian study reports that interferon-gamma (IFN-γ) has potential anti-bacterial bioactivity against infection in vitro; however, its capacity in vivo is ambiguous. In this study, we initially constructed targeting and homologous recombination vectors (containing the IFN-γ gene) and then transferred the vectors into goat mammary gland epithelial cells (GMECs). Enzyme digestion and sequencing analysis indicated that the vectors used in this study were built correctly. Subsequently, monoclonal cells were selected using puromycin and the polymerase chain reaction (PCR) test indicated that IFN-γ was correctly inserted downstream of the casein promoter. Monoclonal cells were then assessed for reducible expression, and reverse transcriptase-PCR (RT-PCR) and Western blot tests confirmed that monoclonal cells could express IFN-γ. Finally, anti-bacterial capacity was evaluated using bacterial counts and flow cytometry analysis. Decreased bacterial counts and cell apoptosis rates in transgenic GMECs demonstrated that the secretion of IFN-γ could inhibit bacterial proliferation. Therefore, IFN-γ gene transfection in goat mammary epithelial cells could inhibit bacterial proliferation and reduce the risk of mammary gland infection in goats.
Keywords: IFN-γ; anti-mastitis activity; gene-editing; infection risk; mastitis.
Copyright © 2022 Liu, Zhang, Dong, Li, Ma, Ge, Hu and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Establishment of a Stable β-Casein Protein-Secreted Laoshan Dairy Goat Mammary Epithelial Cell Line.Front Vet Sci. 2020 Aug 13;7:501. doi: 10.3389/fvets.2020.00501. eCollection 2020. Front Vet Sci. 2020. PMID: 32903554 Free PMC article.
-
Inducible expression of enhanced green fluorescent protein by interleukin-1α, interleukin-1β and Toll-like receptor 2 promoters in goat mammary epithelial cells in response to bacterial challenges.Vet J. 2015 Jan;203(1):85-91. doi: 10.1016/j.tvjl.2014.10.029. Epub 2014 Oct 30. Vet J. 2015. PMID: 25496912
-
Goat mammary gland expression of Cecropin B to inhibit bacterial pathogens causing mastitis.Anim Biotechnol. 2013 Jan;24(1):66-78. doi: 10.1080/10495398.2012.745417. Anim Biotechnol. 2013. PMID: 23394371
-
Mammary gland expression of antibacterial peptide genes to inhibit bacterial pathogens causing mastitis.J Dairy Sci. 2007 Nov;90(11):5218-25. doi: 10.3168/jds.2007-0301. J Dairy Sci. 2007. PMID: 17954762 Clinical Trial.
-
Mammary tissue damage during bovine mastitis: causes and control.J Anim Sci. 2008 Mar;86(13 Suppl):57-65. doi: 10.2527/jas.2007-0302. Epub 2007 Sep 4. J Anim Sci. 2008. PMID: 17785603 Review.
Cited by
-
Immunological composition of human milk before and during subclinical and clinical mastitis.Front Immunol. 2025 Jan 17;15:1532432. doi: 10.3389/fimmu.2024.1532432. eCollection 2024. Front Immunol. 2025. PMID: 39896819 Free PMC article.
References
-
- Moyes KM, Drackley JK, Salak-Johnson JL, Morin DE, Hope JC, Loor JJ. Dietary-induced negative energy balance has minimal effects on innate immunity during a Streptococcus uberis mastitis challenge in dairy cows during midlactation. J Dairy Sci. (2009) 92:4301–16. 10.3168/jds.2009-2170 - DOI - PubMed
-
- Cullor JS. The control, treatment, and prevention of the various types of bovine mastitis. Vet Med. (1993) 88:571–9.
LinkOut - more resources
Full Text Sources

