Directed Biosynthesis of New to Nature Alkaloids in a Heterologous Nicotiana benthamiana Expression Host
- PMID: 35812900
- PMCID: PMC9257203
- DOI: 10.3389/fpls.2022.919443
Directed Biosynthesis of New to Nature Alkaloids in a Heterologous Nicotiana benthamiana Expression Host
Abstract
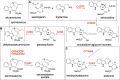
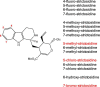
Plants produce a wide variety of pharmacologically active molecules classified as natural products. Derivatization of these natural products can modulate or improve the bioactivity of the parent compound. Unfortunately, chemical derivatization of natural products is often difficult or impractical. Here we use the newly discovered biosynthetic genes for two monoterpene indole alkaloids, alstonine and stemmadenine acetate, to generate analogs of these compounds. We reconstitute these biosynthetic genes in the heterologous host Nicotiana benthamiana along with an unnatural starting substrate to produce the corresponding new-to-nature alkaloid product.
Keywords: Nicotiana benthamiana; alstonine; directed biosynthesis; monoterpene indole alkaloid; natural product; new-to-nature products; stemmadenine.
Copyright © 2022 Boccia, Grzech, Lopes, O’Connor and Caputi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Advances in Metabolic Engineering of Plant Monoterpene Indole Alkaloids.Biology (Basel). 2023 Jul 27;12(8):1056. doi: 10.3390/biology12081056. Biology (Basel). 2023. PMID: 37626942 Free PMC article. Review.
-
Reconstitution of monoterpene indole alkaloid biosynthesis in genome engineered Nicotiana benthamiana.Commun Biol. 2022 Sep 10;5(1):949. doi: 10.1038/s42003-022-03904-w. Commun Biol. 2022. PMID: 36088516 Free PMC article.
-
Chemistry, bioactivity, biosynthesis, and total synthesis of stemmadenine alkaloids.Nat Prod Rep. 2023 May 24;40(5):1022-1044. doi: 10.1039/d2np00052k. Nat Prod Rep. 2023. PMID: 36728407 Review.
-
Nicotiana benthamiana as a Transient Expression Host to Produce Auxin Analogs.Front Plant Sci. 2020 Nov 20;11:581675. doi: 10.3389/fpls.2020.581675. eCollection 2020. Front Plant Sci. 2020. PMID: 33329644 Free PMC article.
-
Silencing of tryptamine biosynthesis for production of nonnatural alkaloids in plant culture.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13673-8. doi: 10.1073/pnas.0903393106. Epub 2009 Aug 5. Proc Natl Acad Sci U S A. 2009. PMID: 19666570 Free PMC article.
Cited by
-
Advances in Metabolic Engineering of Plant Monoterpene Indole Alkaloids.Biology (Basel). 2023 Jul 27;12(8):1056. doi: 10.3390/biology12081056. Biology (Basel). 2023. PMID: 37626942 Free PMC article. Review.
-
Navigating Amaryllidaceae alkaloids: bridging gaps and charting biosynthetic territories.J Exp Bot. 2025 Jan 1;76(1):16-34. doi: 10.1093/jxb/erae187. J Exp Bot. 2025. PMID: 38652148 Free PMC article. Review.
-
Elucidating the enzyme network driving Amaryllidaceae alkaloids biosynthesis in Leucojum aestivum.Plant Biotechnol J. 2025 Jun;23(6):1988-2005. doi: 10.1111/pbi.70026. Epub 2025 Mar 4. Plant Biotechnol J. 2025. PMID: 40035234 Free PMC article.
-
Efficient Agrobacterium-Mediated Methods for Transient and Stable Transformation in Common and Tartary Buckwheat.Int J Mol Sci. 2025 May 6;26(9):4425. doi: 10.3390/ijms26094425. Int J Mol Sci. 2025. PMID: 40362662 Free PMC article.
-
Synthetic Biology in Natural Product Biosynthesis.Chem Rev. 2025 Apr 9;125(7):3814-3931. doi: 10.1021/acs.chemrev.4c00567. Epub 2025 Mar 21. Chem Rev. 2025. PMID: 40116601 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

