KLU/CYP78A5, a Cytochrome P450 Monooxygenase Identified via Fox Hunting, Contributes to Cuticle Biosynthesis and Improves Various Abiotic Stress Tolerances
- PMID: 35812904
- PMCID: PMC9262146
- DOI: 10.3389/fpls.2022.904121
KLU/CYP78A5, a Cytochrome P450 Monooxygenase Identified via Fox Hunting, Contributes to Cuticle Biosynthesis and Improves Various Abiotic Stress Tolerances
Abstract
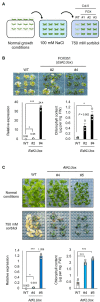
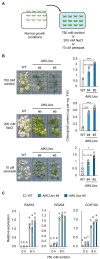
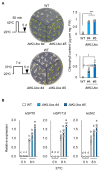
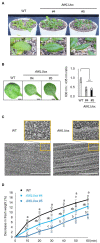
Acquired osmotolerance after salt stress is widespread among Arabidopsis thaliana (Arabidopsis) accessions. Most salt-tolerant accessions exhibit acquired osmotolerance, whereas Col-0 does not. To identify genes that can confer acquired osmotolerance to Col-0 plants, we performed full-length cDNA overexpression (FOX) hunting using full-length cDNAs of halophyte Eutrema salsugineum, a close relative of Arabidopsis. We identified EsCYP78A5 as a gene that can confer acquired osmotolerance to Col-0 wild-type (WT) plants. EsCYP78A5 encodes a cytochrome P450 monooxygenase and the Arabidopsis ortholog is known as KLU. We also demonstrated that transgenic Col-0 plants overexpressing AtKLU (AtKLUox) exhibited acquired osmotolerance. Interestingly, KLU overexpression improved not only acquired osmotolerance but also osmo-shock, salt-shock, oxidative, and heat-stress tolerances. Under normal conditions, the AtKLUox plants showed growth retardation with shiny green leaves. The AtKLUox plants also accumulated higher anthocyanin levels and developed denser cuticular wax than WT plants. Compared to WT plants, the AtKLUox plants accumulated significantly higher levels of cutin monomers and very-long-chain fatty acids, which play an important role in the development of cuticular wax and membrane lipids. Endoplasmic reticulum (ER) stress induced by osmotic or heat stress was reduced in AtKLUox plants compared to WT plants. These findings suggest that KLU is involved in the cuticle biosynthesis, accumulation of cuticular wax, and reduction of ER stress induced by abiotic stresses, leading to the observed abiotic stress tolerances.
Keywords: CYP78A5; cuticle biosynthesis; cuticular wax; heat tolerance; osmotic tolerance.
Copyright © 2022 Kajino, Yamaguchi, Oshima, Nakamura, Narushima, Yaguchi, Yotsui, Sakata and Taji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
ECERIFERUM 10 Encoding an Enoyl-CoA Reductase Plays a Crucial Role in Osmotolerance and Cuticular Wax Loading in Arabidopsis.Front Plant Sci. 2022 Jun 23;13:898317. doi: 10.3389/fpls.2022.898317. eCollection 2022. Front Plant Sci. 2022. PMID: 35812913 Free PMC article.
-
CSP41b, a protein identified via FOX hunting using Eutrema salsugineum cDNAs, improves heat and salinity stress tolerance in transgenic Arabidopsis thaliana.Biochem Biophys Res Commun. 2015 Aug 14;464(1):318-23. doi: 10.1016/j.bbrc.2015.06.151. Epub 2015 Jun 26. Biochem Biophys Res Commun. 2015. PMID: 26123393
-
The Arabidopsis cytochrome P450 enzyme CYP96A4 is involved in the wound-induced biosynthesis of cuticular wax and cutin monomers.Plant J. 2024 Jun;118(5):1619-1634. doi: 10.1111/tpj.16701. Epub 2024 Mar 8. Plant J. 2024. PMID: 38456566
-
Regulatory mechanisms underlying cuticular wax biosynthesis.J Exp Bot. 2022 May 13;73(9):2799-2816. doi: 10.1093/jxb/erab509. J Exp Bot. 2022. PMID: 35560199 Review.
-
Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species.Plant Cell Rep. 2015 Apr;34(4):557-72. doi: 10.1007/s00299-015-1772-2. Epub 2015 Feb 19. Plant Cell Rep. 2015. PMID: 25693495 Review.
Cited by
-
Domesticating Vigna stipulacea: Chromosome-Level genome assembly reveals VsPSAT1 as a candidate gene decreasing hard-seededness.Front Plant Sci. 2023 Apr 17;14:1119625. doi: 10.3389/fpls.2023.1119625. eCollection 2023. Front Plant Sci. 2023. PMID: 37139108 Free PMC article.
-
ECERIFERUM 10 Encoding an Enoyl-CoA Reductase Plays a Crucial Role in Osmotolerance and Cuticular Wax Loading in Arabidopsis.Front Plant Sci. 2022 Jun 23;13:898317. doi: 10.3389/fpls.2022.898317. eCollection 2022. Front Plant Sci. 2022. PMID: 35812913 Free PMC article.
-
Genome-Wide Identification of the CYP78A Gene Family in Lycium and Functional Characterization of LrCYP78A5.Plants (Basel). 2025 Apr 8;14(8):1152. doi: 10.3390/plants14081152. Plants (Basel). 2025. PMID: 40284040 Free PMC article.
References
-
- Aharoni A., Dixit S., Jetter R., Thoenes E., van Arkel G., Pereira A. (2004). The SHINE Clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16, 2463–2480. doi: 10.1105/tpc.104.022897 - DOI - PMC - PubMed
-
- Ariga H., Tanaka T., Ono H., Sakata Y., Hayashi T., Taji T. (2015). CSP41b, a protein identified via FOX hunting using E. salsugineum cDNAs, improves heat and salinity stress tolerance in transgenic A. thaliana. Biochem. Biophys. Res. Commun. 464, 318–323. doi: 10.1016/j.bbrc.2015.06.151 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

