ECERIFERUM 10 Encoding an Enoyl-CoA Reductase Plays a Crucial Role in Osmotolerance and Cuticular Wax Loading in Arabidopsis
- PMID: 35812913
- PMCID: PMC9259793
- DOI: 10.3389/fpls.2022.898317
ECERIFERUM 10 Encoding an Enoyl-CoA Reductase Plays a Crucial Role in Osmotolerance and Cuticular Wax Loading in Arabidopsis
Abstract
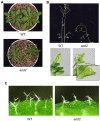
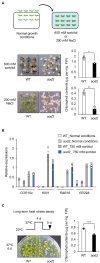
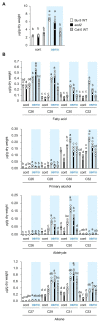
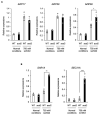
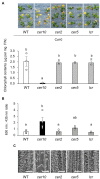
Acquired osmotolerance induced after salt stress is widespread across Arabidopsis thaliana (Arabidopsis) accessions (e.g., Bu-5). However, it remains unclear how this osmotolerance is established. Here, we isolated a mutant showing an acquired osmotolerance-defective phenotype (aod2) from an ion-beam-mutagenized M2 population of Bu-5. aod2 was impaired not only in acquired osmotolerance but also in osmo-shock, salt-shock, and long-term heat tolerances compared with Bu-5, and it displayed abnormal morphology, including small, wrinkled leaves, and zigzag-shaped stems. Genetic analyses of aod2 revealed that a 439-kbp region of chromosome 4 was translocated to chromosome 3 at the causal locus for the osmosensitive phenotype. The causal gene of the aod2 phenotype was identical to ECERIFERUM 10 (CER10), which encodes an enoyl-coenzyme A reductase that is involved in the elongation reactions of very-long-chain fatty acids (VLCFAs) for subsequent derivatization into cuticular waxes, storage lipids, and sphingolipids. The major components of the cuticular wax were accumulated in response to osmotic stress in both Bu-5 WT and aod2. However, less fatty acids, primary alcohols, and aldehydes with chain length ≥ C30 were accumulated in aod2. In addition, aod2 exhibited a dramatic reduction in the number of epicuticular wax crystals on its stems. Endoplasmic reticulum stress mediated by bZIP60 was increased in aod2 under osmotic stress. The only cer10 showed the most pronounced loss of epidermal cuticular wax and most osmosensitive phenotype among four Col-0-background cuticular wax-related mutants. Together, the present findings suggest that CER10/AOD2 plays a crucial role in Arabidopsis osmotolerance through VLCFA metabolism involved in cuticular wax formation and endocytic membrane trafficking.
Keywords: Arabidopsis thaliana accession; ER stress; cuticular wax; enoyl-CoA reductase; osmotolerance; very-long-chain fatty acid.
Copyright © 2022 Fukuda, Oshima, Ariga, Kajino, Koyama, Yaguchi, Tanaka, Yotsui, Sakata and Taji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
KLU/CYP78A5, a Cytochrome P450 Monooxygenase Identified via Fox Hunting, Contributes to Cuticle Biosynthesis and Improves Various Abiotic Stress Tolerances.Front Plant Sci. 2022 Jun 23;13:904121. doi: 10.3389/fpls.2022.904121. eCollection 2022. Front Plant Sci. 2022. PMID: 35812904 Free PMC article.
-
CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme.Plant Cell. 1999 May;11(5):825-38. doi: 10.1105/tpc.11.5.825. Plant Cell. 1999. PMID: 10330468 Free PMC article.
-
Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress.Plant J. 2009 Nov;60(3):462-75. doi: 10.1111/j.1365-313X.2009.03973.x. Epub 2009 Jul 8. Plant J. 2009. PMID: 19619160
-
[Progress in the study on genes encoding enzymes involved in biosynthesis of very long chain fatty acids and cuticular wax in plants].Yi Chuan. 2008 May;30(5):561-7. doi: 10.3724/sp.j.1005.2008.00561. Yi Chuan. 2008. PMID: 18487144 Review. Chinese.
-
Biosynthesis and secretion of plant cuticular wax.Prog Lipid Res. 2003 Jan;42(1):51-80. doi: 10.1016/s0163-7827(02)00045-0. Prog Lipid Res. 2003. PMID: 12467640 Review.
Cited by
-
KLU/CYP78A5, a Cytochrome P450 Monooxygenase Identified via Fox Hunting, Contributes to Cuticle Biosynthesis and Improves Various Abiotic Stress Tolerances.Front Plant Sci. 2022 Jun 23;13:904121. doi: 10.3389/fpls.2022.904121. eCollection 2022. Front Plant Sci. 2022. PMID: 35812904 Free PMC article.
-
MOS4-associated complex contributes to proper splicing and suppression of ER stress under long-term heat stress in Arabidopsis.PNAS Nexus. 2023 Nov 14;2(11):pgad329. doi: 10.1093/pnasnexus/pgad329. eCollection 2023 Nov. PNAS Nexus. 2023. PMID: 38024402 Free PMC article.
-
Genome-Wide Identification of the CER Gene Family and Significant Features in Climate Adaptation of Castanea mollissima.Int J Mol Sci. 2022 Dec 19;23(24):16202. doi: 10.3390/ijms232416202. Int J Mol Sci. 2022. PMID: 36555843 Free PMC article.
-
Evolution, classification, structure, and functional diversification of steroid 5α-reductase family in eukaryotes.Heliyon. 2024 Jul 8;10(14):e34322. doi: 10.1016/j.heliyon.2024.e34322. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108866 Free PMC article. Review.
-
Domesticating Vigna stipulacea: Chromosome-Level genome assembly reveals VsPSAT1 as a candidate gene decreasing hard-seededness.Front Plant Sci. 2023 Apr 17;14:1119625. doi: 10.3389/fpls.2023.1119625. eCollection 2023. Front Plant Sci. 2023. PMID: 37139108 Free PMC article.
References
-
- Ariga H., Tanaka T., Ono H., Sakata Y., Hayashi T., Taji T. (2015). CSP41b, a protein identified via FOX hunting using Eutrema salsugineum cDNAs, improves heat and salinity stress tolerance in transgenic Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 464, 318–323. doi: 10.1016/j.bbrc.2015.06.151, PMID: - DOI - PubMed
-
- Bach L., Michaelson L. V., Haslam R., Bellec Y., Gissot L., Marion J., et al. . (2008). The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proc. Natl. Acad. Sci. U.S.A. 105, 14727–14731. doi: 10.1073/pnas.0805089105, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

