Discriminative Long-Distance Transport of Selenate and Selenite Triggers Glutathione Oxidation in Specific Subcellular Compartments of Root and Shoot Cells in Arabidopsis
- PMID: 35812960
- PMCID: PMC9263558
- DOI: 10.3389/fpls.2022.894479
Discriminative Long-Distance Transport of Selenate and Selenite Triggers Glutathione Oxidation in Specific Subcellular Compartments of Root and Shoot Cells in Arabidopsis
Abstract
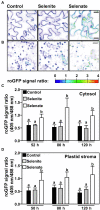
Selenium is an essential trace element required for seleno-protein synthesis in many eukaryotic cells excluding higher plants. However, a substantial fraction of organically bound selenide in human nutrition is directly or indirectly derived from plants, which assimilate inorganic selenium into organic seleno-compounds. In humans, selenium deficiency is associated with several health disorders Despite its importance for human health, selenium assimilation and metabolism is barely understood in plants. Here, we analyzed the impact of the two dominant forms of soil-available selenium, selenite and selenate, on plant development and selenium partitioning in plants. We found that the reference plant Arabidopsis thaliana discriminated between selenate and selenite application. In contrast to selenite, selenate was predominantly deposited in leaves. This explicit deposition of selenate caused chlorosis and impaired plant morphology, which was not observed upon selenite application. However, only selenate triggered the accumulation of the macronutrient sulfur, the sister element of selenium in the oxygen group. To understand the oxidation state-specific toxicity mechanisms for selenium in plants, we quantified the impact of selenate and selenite on the redox environment in the plastids and the cytosol in a time-resolved manner. Surprisingly, we found that selenite first caused the oxidation of the plastid-localized glutathione pool and had a marginal impact on the redox state of the cytosolic glutathione pool, specifically in roots. In contrast, selenate application caused more vigorous oxidation of the cytosolic glutathione pool but also impaired the plastidic redox environment. In agreement with the predominant deposition in leaves, the selenate-induced oxidation of both glutathione pools was more pronounced in leaves than in roots. Our results demonstrate that Se-species dependent differences in Se partitioning substantially contribute to whole plant Se toxicity and that these Se species have subcellular compartment-specific impacts on the glutathione redox buffer that correlate with toxicity symptoms.
Keywords: compartmentation; oxidation; roGFP2; selenium; toxicity.
Copyright © 2022 Khan, Soyk, Wolf, Peter, Meyer, Rausch, Wirtz and Hell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Differences in uptake and translocation of selenate and selenite by the weeping willow and hybrid willow.Environ Sci Pollut Res Int. 2008 Sep;15(6):499-508. doi: 10.1007/s11356-008-0036-x. Epub 2008 Aug 22. Environ Sci Pollut Res Int. 2008. PMID: 18719961
-
Variation in selenium tolerance and accumulation among 19 Arabidopsis thaliana accessions.J Plant Physiol. 2007 Mar;164(3):327-36. doi: 10.1016/j.jplph.2006.01.008. Epub 2006 Mar 2. J Plant Physiol. 2007. PMID: 16513208
-
Comparative responses of cadmium accumulation and subcellular distribution in wheat and rice supplied with selenite or selenate.Environ Sci Pollut Res Int. 2021 Sep;28(33):45075-45086. doi: 10.1007/s11356-021-13554-w. Epub 2021 Apr 15. Environ Sci Pollut Res Int. 2021. PMID: 33855664
-
Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se.Food Addit Contam. 2002 Oct;19(10):974-83. doi: 10.1080/02652030210153578. Food Addit Contam. 2002. PMID: 12443560 Review.
-
[Progress in research of the product of the red elemental selenium reduced from selenium oxyanions by bacteria].Wei Sheng Wu Xue Bao. 2007 Jun;47(3):554-7. Wei Sheng Wu Xue Bao. 2007. PMID: 17672326 Review. Chinese.
Cited by
-
Non-Targeted Metabolome Analysis with Low-Dose Selenate-Treated Arabidopsis.Plants (Basel). 2025 Jan 22;14(3):322. doi: 10.3390/plants14030322. Plants (Basel). 2025. PMID: 39942884 Free PMC article.
-
A Ratiometric Fluorescence Method Based on PCN-224-DABA for the Detection of Se(IV) and Fe(III).Biosensors (Basel). 2024 Dec 19;14(12):626. doi: 10.3390/bios14120626. Biosensors (Basel). 2024. PMID: 39727891 Free PMC article.
References
-
- Arvy M. (1993). Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris). J. Exp. Bot. 44, 1083–1087. doi: 10.1093/jxb/44.6.1083 - DOI
-
- Buchner P., Stuiver C. E. E., Westerman S., Wirtz M., Hell R., Hawkesford M. J., et al. . (2004). Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H2S and pedospheric sulfate nutrition. Plant Physiol. 136, 3396–3408. doi: 10.1104/pp.104.046441, PMID: - DOI - PMC - PubMed
-
- Creissen G., Firmin J., Fryer M., Kular B., Leyland N., Reynolds H., et al. . (1999). Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11, 1277–1291. doi: 10.1105/tpc.11.7.1277, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

