Structural Organization and Function of the Golgi Ribbon During Cell Division
- PMID: 35813197
- PMCID: PMC9263219
- DOI: 10.3389/fcell.2022.925228
Structural Organization and Function of the Golgi Ribbon During Cell Division
Abstract
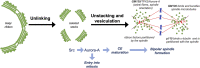
The Golgi complex has a central role in the secretory traffic. In vertebrate cells it is generally organized in polarized stacks of cisternae that are laterally connected by membranous tubules, forming a structure known as Golgi ribbon. The steady state ribbon arrangement results from a dynamic equilibrium between formation and cleavage of the membrane tubules connecting the stacks. This balance is of great physiological relevance as the unlinking of the ribbon during G2 is required for mitotic entry. A block of this process induces a potent G2 arrest of the cell cycle, indicating that a mitotic "Golgi checkpoint" controls the correct pre-mitotic segregation of the Golgi ribbon. Then, after mitosis onset, the Golgi stacks undergo an extensive disassembly, which is necessary for proper spindle formation. Notably, several Golgi-associated proteins acquire new roles in spindle formation and mitotic progression during mitosis. Here we summarize the current knowledge about the basic principle of the Golgi architecture and its functional relationship with cell division to highlight crucial aspects that need to be addressed to help us understand the physiological significance of the ribbon and the pathological implications of alterations of this organization.
Keywords: Golgi ribbon; cancer; checkpoint; mitosis; spindle.
Copyright © 2022 Ayala and Colanzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Golgi checkpoint: Golgi unlinking during G2 is necessary for spindle formation and cytokinesis.Life Sci Alliance. 2024 Mar 13;7(5):e202302469. doi: 10.26508/lsa.202302469. Print 2024 May. Life Sci Alliance. 2024. PMID: 38479814 Free PMC article.
-
Organelle Inheritance Control of Mitotic Entry and Progression: Implications for Tissue Homeostasis and Disease.Front Cell Dev Biol. 2019 Jul 23;7:133. doi: 10.3389/fcell.2019.00133. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31396510 Free PMC article. Review.
-
The Golgi mitotic checkpoint is controlled by BARS-dependent fission of the Golgi ribbon into separate stacks in G2.EMBO J. 2007 May 16;26(10):2465-76. doi: 10.1038/sj.emboj.7601686. Epub 2007 Apr 12. EMBO J. 2007. PMID: 17431394 Free PMC article.
-
In Vitro Methods to Investigate the Disassembly of the Golgi Ribbon During the G2-M Transition of the Cell Cycle.Methods Mol Biol. 2023;2557:333-347. doi: 10.1007/978-1-0716-2639-9_21. Methods Mol Biol. 2023. PMID: 36512225
-
The Golgi ribbon: mechanisms of maintenance and disassembly during the cell cycle.Biochem Soc Trans. 2020 Feb 28;48(1):245-256. doi: 10.1042/BST20190646. Biochem Soc Trans. 2020. PMID: 32010930 Review.
Cited by
-
Dominant ARF3 variants disrupt Golgi integrity and cause a neurodevelopmental disorder recapitulated in zebrafish.Nat Commun. 2022 Nov 11;13(1):6841. doi: 10.1038/s41467-022-34354-x. Nat Commun. 2022. PMID: 36369169 Free PMC article.
-
Editorial: Does the golgi complex enable oncogenesis?Front Cell Dev Biol. 2022 Aug 30;10:1000946. doi: 10.3389/fcell.2022.1000946. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36111334 Free PMC article. No abstract available.
-
Golgi-Targeting Anticancer Natural Products.Cancers (Basel). 2023 Mar 31;15(7):2086. doi: 10.3390/cancers15072086. Cancers (Basel). 2023. PMID: 37046746 Free PMC article. Review.
-
Dynamic regulation of Sec24C by phosphorylation and O-GlcNAcylation during cell cycle progression.J Biol Chem. 2025 Jul 3;301(8):110456. doi: 10.1016/j.jbc.2025.110456. Online ahead of print. J Biol Chem. 2025. PMID: 40617351 Free PMC article.
-
Mitotic spindle membranes.Mol Biol Cell. 2025 Apr 1;36(4):re1. doi: 10.1091/mbc.E24-10-0475. Mol Biol Cell. 2025. PMID: 40067152 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

