Field Evaluation of a Duplex RT-RAA Assay for Rapid Detection of SARS-CoV-2 - Hebei Province, China, January 2021
- PMID: 35813263
- PMCID: PMC9257232
- DOI: 10.46234/ccdcw2022.111
Field Evaluation of a Duplex RT-RAA Assay for Rapid Detection of SARS-CoV-2 - Hebei Province, China, January 2021
Abstract
Introduction: Recently, a local cluster epidemic has occurred in Shijiazhuang City, Hebei Province. Failure to promptly identify patients with fever in rural areas was the major reason for this epidemic.
Methods: We presented the field evaluation of a new real-time reverse transcription recombinase-aided amplification (RT-RAA) kit incorporating an endogenous internal control in a single-tube format, completed at the Hebei CDC from January 17, 2021 to January 27, 2021.
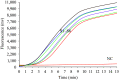
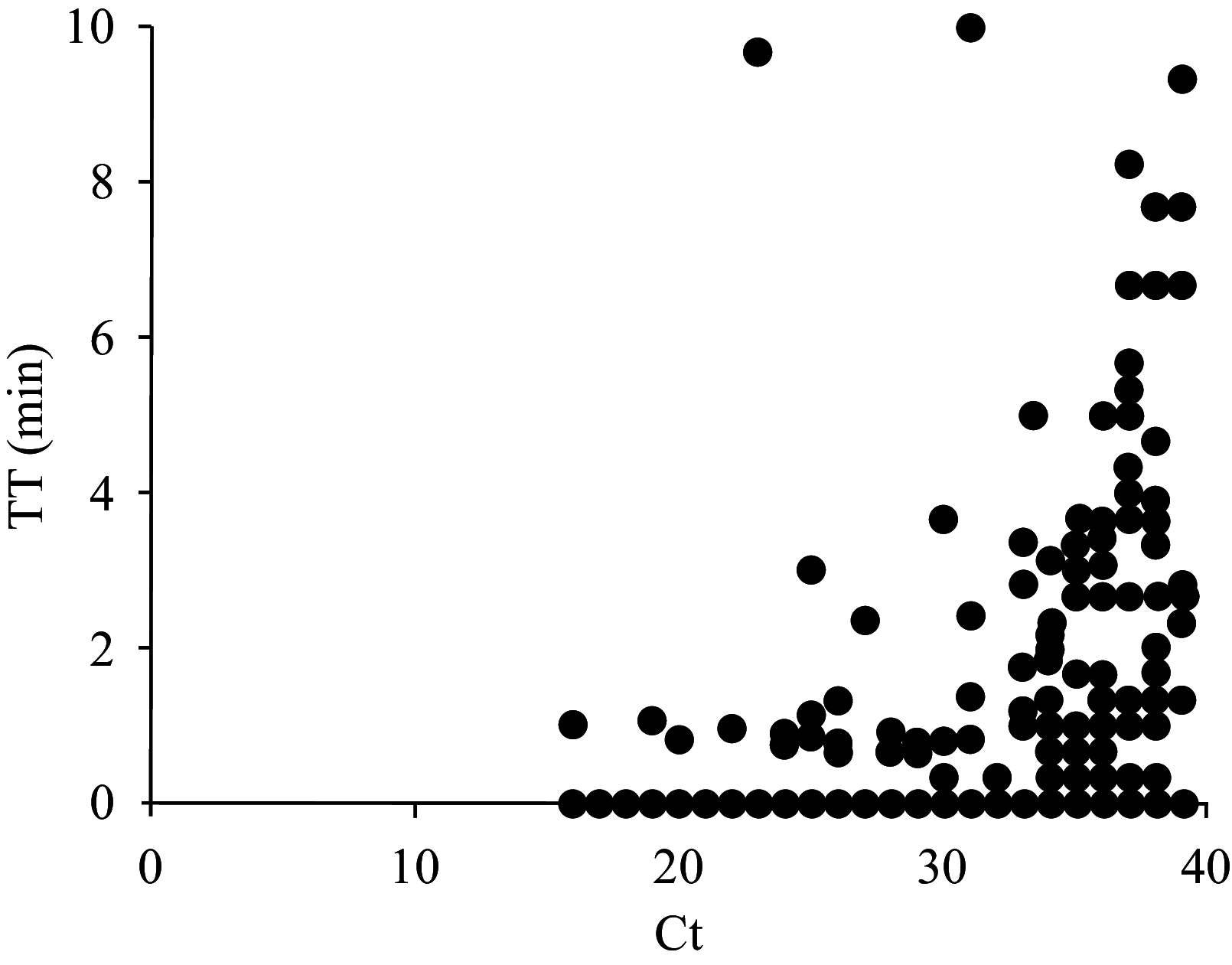
Results: We evaluated the diagnostic performance of RT-RAA assay using automatic extracted RNA of 808 clinical samples. Compared with reverse transcriptase real-time quantitative PCR (qRT-PCR), RT-RAA kit achieved 92.41% sensitivity, 98.78% specificity and a 96.29% coincidence rate, demonstrating an excellent agreement between the RT-RAA assay and qRT-PCR assay. Furthermore, 58 samples were extracted using a manual extraction method within 5 minutes, but only samples with high nucleic acid concentration (cycle threshold value not higher than 32) could be stably detected.
Discussion: The RT-RAA is more suitable to meet the needs of rapid, sensitive, and accurate detection in community-level medical institutions.
Keywords: COVID-19; RT-RAA kit; SARS-CoV-2; the field evaluation.
Copyright and License information: Editorial Office of CCDCW, Chinese Center for Disease Control and Prevention 2022.
Conflict of interest statement
No conflicts of interest declared.
Figures

Similar articles
-
Field Validation of a Rapid Recombinase Aided Amplification Assay for SARS-CoV-2 RNA at Customs - Zhejiang Province, China, January 2021.China CDC Wkly. 2021 Nov 12;3(46):973-976. doi: 10.46234/ccdcw2021.236. China CDC Wkly. 2021. PMID: 34804630 Free PMC article.
-
Multiple-centre clinical evaluation of an ultrafast single-tube assay for SARS-CoV-2 RNA.Clin Microbiol Infect. 2020 Aug;26(8):1076-1081. doi: 10.1016/j.cmi.2020.05.007. Epub 2020 May 15. Clin Microbiol Infect. 2020. PMID: 32422410 Free PMC article.
-
Real-Time Reverse Transcription Recombinase-Aided Amplification Assay for Rapid Amplification of the N Gene of SARS-CoV-2.Int J Mol Sci. 2022 Dec 3;23(23):15269. doi: 10.3390/ijms232315269. Int J Mol Sci. 2022. PMID: 36499594 Free PMC article.
-
Establishment of reverse transcription recombinase-aided amplification-lateral-flow dipstick and real-time fluorescence-based reverse transcription recombinase-aided amplification methods for detection of the Newcastle disease virus in chickens.Poult Sci. 2020 Jul;99(7):3393-3401. doi: 10.1016/j.psj.2020.03.018. Epub 2020 Apr 15. Poult Sci. 2020. PMID: 32616233 Free PMC article.
-
Applicability of duplex real time and lateral flow strip reverse-transcription recombinase aided amplification assays for the detection of Enterovirus 71 and Coxsackievirus A16.Virol J. 2019 Dec 30;16(1):166. doi: 10.1186/s12985-019-1264-z. Virol J. 2019. PMID: 31888694 Free PMC article.
Cited by
-
A duplex one-step recombinase aided PCR assay for the rapid and sensitive detection of the isoniazid resistance genes katG and inhA in Mycobacterium tuberculosis.Front Microbiol. 2025 Mar 13;16:1548965. doi: 10.3389/fmicb.2025.1548965. eCollection 2025. Front Microbiol. 2025. PMID: 40182291 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
