Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): a prospective cohort study
- PMID: 35813279
- PMCID: PMC9252508
- DOI: 10.1016/S2666-7568(22)00147-7
Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): a prospective cohort study
Abstract
Background: Residents and staff in long-term care facilities have been prioritised for vaccination against SARS-CoV-2, but data on potential waning of vaccine effectiveness and the effect of booster doses in this vulnerable population are scarce. We aimed to evaluate effectiveness of one, two, and three vaccine doses against infection and severe clinical outcomes in staff and residents of long-term care facilities in England over the first year following vaccine roll-out.
Methods: The VIVALDI study is a prospective cohort study done in 331 long-term care facilities in England. Residents aged 65 years or older and staff aged 18 years or older were eligible for participation. Participants had routine PCR testing throughout the study period between Dec 8, 2020, and Dec 11, 2021. We retrieved all PCR results and cycle threshold values for PCR-positive samples from routine testing in long-term care facilities, and positive PCR results from clinical testing in hospitals through the UK's COVID-19 Datastore. PCR results were linked to participants using pseudo-identifiers based on individuals' unique UK National Health Service (NHS) numbers, which were also used to retrieve vaccination records from the National Immunisation Management Service, hospitalisation records from NHS England, and deaths data from the Office for National Statistics through the COVID-19 Datastore. In a Cox proportional hazards regression, we estimated vaccine effectiveness against SARS-CoV-2 infection, COVID-19-related hospitalisation, and COVID-19-related death after one, two, and three vaccine doses, separately by previous SARS-CoV-2 exposure. This study is registered with the ISRCTN Registry, ISRCTN 14447421.
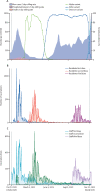
Findings: 80 186 residents and staff of long-term care facilities had records available for the study period, of whom 15 518 eligible residents and 19 515 eligible staff were included in the analysis. For residents without evidence of previous SARS-CoV-2 exposure, vaccine effectiveness decreased from 61·7% (95% CI 35·1 to 77·4) to 22·0% (-14·9 to 47·0) against infection; from 89·0% (70·6 to 95·9) to 56·3% (30·1 to 72·6) against hospitalisation; and from 96·4% (84·3 to 99·2) to 64·4% (36·1 to 80·1) against death, when comparing 14-83 days after dose two and 84 days or more after dose two. For staff without evidence of previous exposure, vaccine effectiveness against infection decreased slightly from 57·9% (43·1 to 68·9) at 14-83 days after dose two to 42·1% (29·9 to 52·2) at 84 days or more after dose two. There were no hospitalisations or deaths among unexposed staff at 14-83 days, but seven hospitalisations (vaccine effectiveness 91·0% [95% CI 74·3 to 96·8]) and one death were observed at 84 days or more after dose two. High vaccine effectiveness was restored following a third vaccine dose, with vaccine effectiveness in unexposed residents of 72·7% (55·8 to 83·1) against infection, 90·1% (80·6 to 95·0) against hospitalisation, and 97·5% (88·1 to 99·5) against death; and vaccine effectiveness in unexposed staff of 78·2% (70·0 to 84·1) against infection and 95·8% (49·9 to 99·6) against hospitalisation. There were no COVID-19-related deaths among unexposed staff after the third vaccine dose.
Interpretation: Our findings showed substantial waning of SARS-CoV-2 vaccine effectiveness against all outcomes in residents of long-term care facilities from 12 weeks after a primary course of ChAdOx1-S or mRNA vaccines. Boosters restored protection, and maximised immunity across all outcomes. These findings show the importance of boosting and the need for ongoing surveillance in this vulnerable cohort.
Funding: UK Government Department of Health and Social Care.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Conflict of interest statement
LS reports grants from the Department of Health and Social Care during the conduct of the study and is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. AI-S and VB are employed by the Department of Health and Social Care who funded the study. AH reports funding from the COVID Core Studies Programme and is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health and Environmental Modelling Group of the Scientific Advisory Group for Emergencies. All other authors declare no competing interests.
Figures
Comment in
-
Dawn of the hybrid immunity era in long-term care facilities.Lancet Healthy Longev. 2022 Jul;3(7):e451-e452. doi: 10.1016/S2666-7568(22)00151-9. Epub 2022 Jul 4. Lancet Healthy Longev. 2022. PMID: 35813278 Free PMC article. No abstract available.
Similar articles
-
Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study.Lancet Infect Dis. 2021 Nov;21(11):1529-1538. doi: 10.1016/S1473-3099(21)00289-9. Epub 2021 Jun 23. Lancet Infect Dis. 2021. PMID: 34174193 Free PMC article.
-
Outcomes of SARS-CoV-2 omicron infection in residents of long-term care facilities in England (VIVALDI): a prospective, cohort study.Lancet Healthy Longev. 2022 May;3(5):e347-e355. doi: 10.1016/S2666-7568(22)00093-9. Epub 2022 May 4. Lancet Healthy Longev. 2022. PMID: 35531432 Free PMC article.
-
Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): an observational study.Lancet Healthy Longev. 2021 Sep;2(9):e544-e553. doi: 10.1016/S2666-7568(21)00168-9. Epub 2021 Aug 19. Lancet Healthy Longev. 2021. PMID: 34430954 Free PMC article.
-
Real-world evidence on the efficacy of bivalent booster doses of SARS-CoV-2 vaccine in respect of monovalent boosters or primary cycle of vaccination: a narrative review.Epidemiol Prev. 2023 Nov-Dec;47(6):331-343. doi: 10.19191/EP23.6.A626.081. Epidemiol Prev. 2023. PMID: 38314543 Review. English.
-
Protection of the third-dose and fourth-dose mRNA vaccines against SARS-CoV-2 Omicron subvariant: a systematic review and meta-analysis.BMJ Open. 2023 Dec 20;13(12):e076892. doi: 10.1136/bmjopen-2023-076892. BMJ Open. 2023. PMID: 38128943 Free PMC article.
Cited by
-
COVID-19 outbreak in an elderly care home: Very low vaccine effectiveness and late impact of booster vaccination campaign.Vaccine. 2022 Nov 2;40(46):6664-6669. doi: 10.1016/j.vaccine.2022.09.080. Epub 2022 Oct 4. Vaccine. 2022. PMID: 36216647 Free PMC article.
-
How do large-scale population studies inform vaccine evaluations in England?Clin Exp Immunol. 2025 Jan 21;219(1):uxaf006. doi: 10.1093/cei/uxaf006. Clin Exp Immunol. 2025. PMID: 39910973 Review.
-
[Effect of previous exposure to COVID-19, occurrence of spikes, and type of vaccine on the humoral immune response of institutionalized older adults].Cad Saude Publica. 2024 Oct 11;40(9):e00155023. doi: 10.1590/0102-311XES155023. eCollection 2024. Cad Saude Publica. 2024. PMID: 39417469 Free PMC article. Spanish.
-
The impact of dementia, frailty and care home characteristics on SARS-CoV-2 incidence in a national cohort of Welsh care home residents during a period of high community prevalence.Age Ageing. 2022 Dec 5;51(12):afac250. doi: 10.1093/ageing/afac250. Age Ageing. 2022. PMID: 36469089 Free PMC article.
-
COVID-19 Vaccine Effectiveness of Booster Doses Against Delta and Omicron Variants Over Follow-up Times Using Longitudinal Meta-analysis.J Res Health Sci. 2024 Sep 30;24(4):e00626. doi: 10.34172/jrhs.2024.161. Epub 2024 Sep 30. J Res Health Sci. 2024. PMID: 39431651 Free PMC article.
References
-
- Castro-Herrera VM, Lown M, Fisk HL, et al. Relationships between age, frailty, length of care home residence and biomarkers of immunity and inflammation in older care home residents in the United Kingdom. Front Aging. 2021 doi: 10.3389/fragi.2021.599084. published online March 17. - DOI - PMC - PubMed
-
- Department of Health and Social Care Independent report—priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI. 30 December 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavir...
-
- Department of Health and Social Care Independent report—JCVI statement, September 2021: COVID-19 booster vaccine programme for winter 2021 to 2022. https://www.gov.uk/government/publications/jcvi-statement-september-2021...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

