Ventrolateral Periaqueductal Gray Neurons Are Active During Urination
- PMID: 35813503
- PMCID: PMC9259957
- DOI: 10.3389/fncel.2022.865186
Ventrolateral Periaqueductal Gray Neurons Are Active During Urination
Abstract
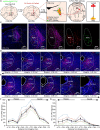
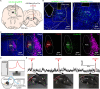
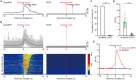
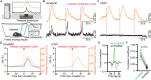
The ventrolateral periaqueductal gray (VLPAG) is thought to be the main PAG column for bladder control. PAG neurons (especially VLPAG neurons) and neurons in the pontine micturition center (PMC) innervating the bladder detrusor have anatomical and functional synaptic connections. The prevailing viewpoint on neural control of the bladder is that PAG neurons receive information on the decision to void made by upstream brain regions, and consequently activate the PMC through their direct projections to initiate urination reflex. However, the exact location of the PMC-projecting VLPAG neurons, their activity in response to urination, and their whole-brain inputs remain unclear. Here, we identified the distribution of VLPAG neurons that may participate in control of the bladder or project to the PMC through retrograde neural tracing. Population Ca2+ signals of PMC-projecting VLPAG neurons highly correlated with bladder contractions and urination as shown by in vivo recording in freely moving animals. Using a RV-based retrograde trans-synaptic tracing strategy, morphological results showed that urination-related PMC-projecting VLPAG neurons received dense inputs from multiple urination-related higher brain areas, such as the medial preoptic area, medial prefrontal cortex, and lateral hypothalamus. Thus, our findings reveal a novel insight into the VLPAG for control of bladder function and provide a potential therapeutic midbrain node for neurogenic bladder dysfunction.
Keywords: cystometry; fiber photometry; pontine micturition center; rabies virus; urination; ventrolateral periaqueductal gray (vlPAG).
Copyright © 2022 Rao, Gao, Li, Li, Li, Liang, Li, Zhai, Yan, Yao and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
SST neurons in the periaqueductal gray regulate urination and bladder function.Commun Biol. 2025 Apr 21;8(1):639. doi: 10.1038/s42003-025-08069-w. Commun Biol. 2025. PMID: 40259086 Free PMC article.
-
Micturition and the soul.J Comp Neurol. 2005 Dec 5;493(1):15-20. doi: 10.1002/cne.20785. J Comp Neurol. 2005. PMID: 16254993 Review.
-
Electrophysiological responses of the ventrolateral periaqueductal gray matter neurons towards peripheral bladder stimulation.Brain Res Bull. 2018 Sep;142:116-121. doi: 10.1016/j.brainresbull.2018.07.009. Epub 2018 Jul 18. Brain Res Bull. 2018. PMID: 30016723
-
Activation of mu opioid receptors in the ventrolateral periaqueductal gray inhibits reflex micturition in anesthetized rats.Neurosci Lett. 2004 Jun 10;363(2):116-9. doi: 10.1016/j.neulet.2004.03.055. Neurosci Lett. 2004. PMID: 15172097
-
The emotional motor system and micturition control.Neurourol Urodyn. 2010;29(1):42-8. doi: 10.1002/nau.20789. Neurourol Urodyn. 2010. PMID: 20025036 Review.
Cited by
-
Enkephalinergic Neurons in Barrington's Nucleus Gate Sex-Specific Control of Micturition.Res Sq [Preprint]. 2025 Jul 2:rs.3.rs-6940959. doi: 10.21203/rs.3.rs-6940959/v1. Res Sq. 2025. PMID: 40630540 Free PMC article. Preprint.
-
Periaqueductal gray subregions connectivity and its association with micturition desire-awakening function.Eur Child Adolesc Psychiatry. 2025 Apr;34(4):1435-1444. doi: 10.1007/s00787-024-02574-9. Epub 2024 Sep 5. Eur Child Adolesc Psychiatry. 2025. PMID: 39235463
-
SST neurons in the periaqueductal gray regulate urination and bladder function.Commun Biol. 2025 Apr 21;8(1):639. doi: 10.1038/s42003-025-08069-w. Commun Biol. 2025. PMID: 40259086 Free PMC article.
-
Noradrenergic Pathways Involved in Micturition in an Animal Model of Hydrocephalus-Implications for Urinary Dysfunction.Biomedicines. 2024 Jan 18;12(1):215. doi: 10.3390/biomedicines12010215. Biomedicines. 2024. PMID: 38255319 Free PMC article.
-
The GPR39 Receptor Plays an Important Role in the Pathogenesis of Overactive Bladder and Corticosterone-Induced Depression.Int J Mol Sci. 2024 Nov 25;25(23):12630. doi: 10.3390/ijms252312630. Int J Mol Sci. 2024. PMID: 39684342 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

