Safety of Intraoperative Cell Salvage in Cancer Surgery: An Updated Meta-Analysis of the Current Literature
- PMID: 35813601
- PMCID: PMC9210012
- DOI: 10.1159/000524538
Safety of Intraoperative Cell Salvage in Cancer Surgery: An Updated Meta-Analysis of the Current Literature
Abstract
Background: Allogeneic blood transfusions in oncologic surgery are associated with increased recurrence and mortality. Adverse effects on outcome could be reduced or avoided by using intraoperative autologous blood cell salvage (IOCS). However, there are concerns regarding the safety of the autologous IOCS blood. Previous meta-analyses from 2012 and 2020 did not identify increased risk of cancer recurrence after using autologous IOCS blood. The objective of this review was to reassess a greater number of IOCS-treated patients to present an updated and more robust analysis of the current literature.
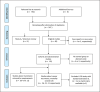
Methods: This systematic review includes full-text articles listed in PubMed, Cochrane, Cochrane Reviews, and Web of Science. We analyzed publications that discussed cell salvage or autotransfusion combined with the following outcomes: cancer recurrence, mortality, survival, allogeneic transfusion rate and requirements, length of hospital stay (LOS). To rate the strength of evidence, a Grading of Recommendations Assessment, Development and Evaluation (GRADE) of the underlying evidence was applied.
Results: In the updated meta-analysis, 7 further observational studies were added to the original 27 observational studies included in the former 2020 analysis. Studies compared either unfiltered (n = 2,311) or filtered (n = 850) IOCS (total n = 3,161) versus non-IOCS use (n = 5,342). Control patients were either treated with autologous predonated blood (n = 484), with allogeneic transfusion (n = 4,113), or did not receive a blood transfusion (n = 745). However, the current literature still contains only observational studies on these topics, and the strength of evidence remains low. The risk of cancer recurrence was reduced in recipients of autologous salvaged blood with or without LDF (odds ratio [OR] 0.76, 95% confidence interval [CI]: 0.64-0.90) compared to nontransfused patients or patients with allogeneic transfusion. There was no difference in mortality (OR 0.95, 95% CI: 0.71-1.27) and LOS (mean difference -0.07 days, 95% CI: -0.63 to 0.48) between patients treated with IOCS blood or those in whom IOCS was not used. Due to high heterogeneity, transfusion rates or volumes could not be analyzed.
Conclusion: Randomized controlled trials comparing mortality and cancer recurrence rate of IOCS with or without LDF filtration versus allogeneic blood transfusion were not found. Outcome was similar or better in patients receiving IOCS during cancer surgery compared to patients with allogeneic blood transfusion or nontransfused patients.
Keywords: Autologous transfusion; Cancer recurrence; Cell salvage; Tumor surgery.
Copyright © 2022 by S. Karger AG, Basel.
Conflict of interest statement
T.F.: honoraria and reimbursements for travel expenses, lectures, investigator meetings, and presentations from Janssen-Cilag, AstraZeneca, Vifor Pharma, Pharmacosmos, the German Red Cross, Aspect Medical, Organon, Alliance Pharmaceuticals, and Baxter Healthcare Corp; expert consulting contracts with Janssen-Cilag, Vifor Pharma, Pharmacosmos; research grants from the Else-Groenert-Foundation and the University Medicine Mannheim, University of Heidelberg. A.U.S.: research grant from Pharmacosmos, Denmark, to perform a single-center, prospective trial on preoperative anemia treatment. A.U.S. is supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) grant STE 1895/9-1 and STE 1895/10-1 as part of the DFG research consortium FerrOS-FOR5146. A.H.: no conflicts of interest to declare. M.M.: no conflicts of interest to declare. G.D.: no conflicts of interest to declare. M.A.W.: no conflicts of interest to declare. J.H.W.: no conflicts of interest to declare. D.F.: no conflicts of interest to declare.
Figures





References
-
- Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–44. - PubMed
-
- Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2015;13:102–10. - PubMed
LinkOut - more resources
Full Text Sources

