Mitchell-Riley Syndrome: Improving Clinical Outcomes and Searching for Functional Impact of RFX-6 Mutations
- PMID: 35813646
- PMCID: PMC9257252
- DOI: 10.3389/fendo.2022.802351
Mitchell-Riley Syndrome: Improving Clinical Outcomes and Searching for Functional Impact of RFX-6 Mutations
Abstract
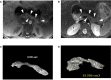
Aims/hypothesis: Caused by biallelic mutations of the gene encoding the transcription factor RFX6, the rare Mitchell-Riley syndrome (MRS) comprises neonatal diabetes, pancreatic hypoplasia, gallbladder agenesis or hypoplasia, duodenal atresia, and severe chronic diarrhea. So far, sixteen cases have been reported, all with a poor prognosis. This study discusses the multidisciplinary intensive clinical management of 4 new cases of MRS that survived over the first 2 years of life. Moreover, it demonstrates how the mutations impair the RFX6 function.
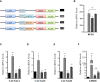
Methods: Clinical records were analyzed and described in detail. The functional impact of two RFX6R181W and RFX6V506G variants was assessed by measuring their ability to transactivate insulin transcription and genes that encode the L-type calcium channels required for normal pancreatic beta-cell function.
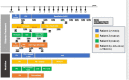
Results: All four patients were small for gestational age (SGA) and prenatally diagnosed with duodenal atresia. They presented with neonatal diabetes early in life and were treated with intravenous insulin therapy before switching to subcutaneous insulin pump therapy. All patients faced recurrent hypoglycemic episodes, exacerbated when parenteral nutrition (PN) was disconnected. A sensor-augmented insulin pump therapy with a predictive low-glucose suspension system was installed with good results. One patient had a homozygous c.1517T>G (p.Val506Gly) mutation, two patients had a homozygous p.Arg181Trp mutation, and one patient presented with new compound heterozygosity. The RFX6V506G and RFX6R181W mutations failed to transactivate the expression of insulin and genes that encode L-type calcium channel subunits required for normal pancreatic beta-cell function.
Conclusions/interpretation: Multidisciplinary and intensive disease management improved the clinical outcomes in four patients with MRS, including adjustment of parenteral/oral nutrition progression and advanced diabetes technologies. A better understanding of RFX6 function, in both intestine and pancreas cells, may break ground in new therapies, particularly regarding the use of drugs that modulate the enteroendocrine system.
Keywords: Mitchell–Riley syndrome; RFX6; beta-cell function; diabetes technology; neonatal diabetes mellitus; parenteral nutrition.
Copyright © 2022 Passone, Vermillac, Staels, Besancon, Kariyawasam, Godot, Lambe, Talbotec, Girard, Chardot, Berteloot, Hachem, Lapillonne, Poidvin, Storey, Neve, Stan, Dugelay, Fauret-Amsellem, Capri, Cavé, Ybarra, Chandra, Scharfmann, Bismuth, Polak, Carel, Pigneur and Beltrand.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Mitchell J, Punthakee Z, Lo B, Bernard C, Chong K, Newman C, et al. . Neonatal Diabetes, With Hypoplastic Pancreas, Intestinal Atresia and Gall Bladder Hypoplasia: Search for the Aetiology of a New Autosomal Recessive Syndrome. Diabetologia (2004) 47:2160–7. doi: 10.1007/s00125-004-1576-3 - DOI - PubMed

