A novel angiographic classification of pseudoaneurysms of the pulmonary chronic inflammatory cavity based on selective angiograms and therapeutic implications
- PMID: 35813718
- PMCID: PMC9264097
- DOI: 10.21037/jtd-21-1485
A novel angiographic classification of pseudoaneurysms of the pulmonary chronic inflammatory cavity based on selective angiograms and therapeutic implications
Abstract
Background: Hemoptysis is a common clinical symptom. In the chronic tuberculosis cavity and chronic necrotizing pneumonia cavity, pseudoaneurysms (Pas) easily form and are prone to massive hemoptysis and repeated hemoptysis and can even endanger patient's life. However, it remains to be further analyzed whether Pas of the pulmonary chronic inflammatory cavity selectively affect the peripheral pulmonary branches. This study is based on selective angiography to classify peripheral pulmonary arterial Pas (PAPs) of the pulmonary chronic inflammatory cavity and to determine treatment options for PAPs, thereby guiding individualized clinical treatment.
Methods: Angiographic data of 392 noncancer patients undergoing hemoptysis were retrospectively analyzed. All of the patients underwent pulmonary and selective pulmonary angiography and bronchial and nonbronchial systemic collateral arterial angiography. A total of 9 patients had Pas of the pulmonary chronic inflammatory cavity, and a pseudoaneurysm systemic artery collateral (Pasac), inflow and outflow sections of the parent vessels, and direction of blood flow in the parent vessels were clearly observed with digital subtraction angiography (DSA) and/or C-arm cone-beam flat-panel detector computed tomography angiography (CBCTA). Patients with underlying disease had pulmonary tuberculosis (n=8) or lung abscess (n=1). The angiographic types of Pas were analyzed.
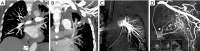
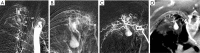
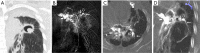
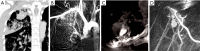
Results: Eight patients with chronic pulmonary tuberculosis and 1 patient with a necrotizing pneumonia cavity in the convalescent period were included in the study. Pas of the pulmonary chronic inflammatory cavity presented the following types: (I) pulmonary artery pseudoaneurysm (PAPa) (n=2 cases); (II) body arterial Pa (n=3 cases); and (III) systemic-pulmonary anastomosis Pa. Each type could be divided into two subtypes (n=4 cases). In nine cases, embolization and hemostasis were technically and clinically successful.
Conclusions: Pas of the pulmonary chronic inflammatory cavity are diverse (especially in cases of pulmonary tuberculosis). Angiographic typing plays a guiding role in the selection of an embolization strategy.
Keywords: Pseudoaneurysm (Pa); angiography; cavity; embolization; hemoptysis.
2022 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1485/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Peripheral pulmonary arterial pseudoaneurysms: therapeutic implications of endovascular treatment and angiographic classifications.Radiology. 2010 Aug;256(2):656-64. doi: 10.1148/radiol.10091416. Radiology. 2010. PMID: 20656846
-
Therapeutic approaches for pulmonary artery pseudoaneurysms and analysis of outcomes.Eur Radiol. 2025 Jun 14. doi: 10.1007/s00330-025-11749-0. Online ahead of print. Eur Radiol. 2025. PMID: 40515770
-
[Endovascular embolization for pulmonary artery pseudoaneurysms with massive hemoptysis].Zhonghua Jie He He Hu Xi Za Zhi. 2020 Mar 12;43(3):223-227. doi: 10.3760/cma.j.issn.1001-0939.2020.03.017. Zhonghua Jie He He Hu Xi Za Zhi. 2020. PMID: 32164093 Chinese.
-
Successful embolization in childhood hemoptysis due to abnormal systemic arterial bleeding of the lung and review of the literature.Clin Respir J. 2016 Nov;10(6):693-697. doi: 10.1111/crj.12289. Epub 2015 Apr 6. Clin Respir J. 2016. PMID: 25773166 Review.
-
Multi-detector row CT of hemoptysis.Radiographics. 2006 Jan-Feb;26(1):3-22. doi: 10.1148/rg.261045726. Radiographics. 2006. PMID: 16418239 Review.
References
LinkOut - more resources
Full Text Sources
