Non-Coding RNAs: New Dawn for Diabetes Mellitus Induced Erectile Dysfunction
- PMID: 35813828
- PMCID: PMC9257010
- DOI: 10.3389/fmolb.2022.888624
Non-Coding RNAs: New Dawn for Diabetes Mellitus Induced Erectile Dysfunction
Abstract
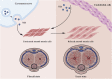
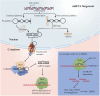
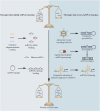
Erectile dysfunction (ED) is a common sexual dysfunction in males, with multifactorial alterations which consist of psychological and organic. Diabetes mellitus (DM) induced erectile dysfunction (DMED) is a disconcerting and critical complication of DM, and remarkably different from non-diabetic ED. The response rate of phosphodiesterase type 5 inhibitor (PDE5i), a milestone for ED therapy, is far from satisfactory in DMED. Unfortunately, the contributing mechanisms of DMED remains vague. Hence, It is urgent to seek for novel prospective biomarkers or targets of DMED. Numerous studies have proved that non-coding RNAs (ncRNAs) play essential roles in the pathogenesis process of DM, which comprise of long non-coding RNAs (lncRNAs) and small non-coding RNAs (sncRNAs) like microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs) and circular RNAs (circRNAs). However, the implications of ncRNAs in DMED are still understudied. This review highlights the pathophysiology of DMED, summarizes identified mechanisms of ncRNAs associated with DMED and covers the topic of perspectives for ncRNAs in DMED.
Keywords: diabetes mellitus; erectile dysfunction; lncRNA; miRNA; non-coding RNA.
Copyright © 2022 Xu, Jiang, Liu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

