Trimeric receptor-binding domain of SARS-CoV-2 acts as a potent inhibitor of ACE2 receptor-mediated viral entry
- PMID: 35813876
- PMCID: PMC9251894
- DOI: 10.1016/j.isci.2022.104716
Trimeric receptor-binding domain of SARS-CoV-2 acts as a potent inhibitor of ACE2 receptor-mediated viral entry
Abstract
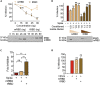
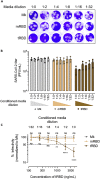
The COVID-19 pandemic has caused over four million deaths and effective methods to control CoV-2 infection, in addition to vaccines, are needed. The CoV-2 binds to the ACE2 on human cells through the receptor-binding domain (RBD) of the trimeric spike protein. Our modeling studies show that a modified trimeric RBD (tRBD) can interact with three ACE2 receptors, unlike the native spike protein, which binds to only one ACE2. We found that tRBD binds to the ACE2 with 58-fold higher affinity than monomeric RBD (mRBD) and blocks spike-dependent pseudoviral infection over 4-fold more effectively compared to the mRBD. Although mRBD failed to block CoV-2 USA-WA1/2020 infection, tRBD efficiently blocked the true virus infection in plaque assays. We show that tRBD is a potent inhibitor of CoV-2 through both competitive binding to the ACE2 and steric hindrance, and has the potential to emerge as a first-line therapeutic method to control COVID-19.
Keywords: Molecular structure; Virology.
© 2022.
Conflict of interest statement
PR has submitted an invention disclosure 2020–3814, on the use of trimeric RBD as a multipotential therapeutic target to Case Western Reserve University. All other authors declare no conflict of interest in this study.
Figures
Similar articles
-
Competitive SARS-CoV-2 Serology Reveals Most Antibodies Targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding.mSphere. 2020 Sep 16;5(5):e00802-20. doi: 10.1128/mSphere.00802-20. mSphere. 2020. PMID: 32938700 Free PMC article.
-
Molecular dynamics simulations and functional studies reveal that hBD-2 binds SARS-CoV-2 spike RBD and blocks viral entry into ACE2 expressing cells.bioRxiv [Preprint]. 2021 Jan 7:2021.01.07.425621. doi: 10.1101/2021.01.07.425621. bioRxiv. 2021. PMID: 33442698 Free PMC article. Preprint.
-
Screening of inhibitors against SARS-CoV-2 spike protein and their capability to block the viral entry mechanism: A viroinformatics study.Saudi J Biol Sci. 2021 Jun;28(6):3262-3269. doi: 10.1016/j.sjbs.2021.02.066. Epub 2021 Feb 26. Saudi J Biol Sci. 2021. PMID: 33654454 Free PMC article.
-
Interactions of angiotensin-converting enzyme-2 (ACE2) and SARS-CoV-2 spike receptor-binding domain (RBD): a structural perspective.Mol Biol Rep. 2023 Mar;50(3):2713-2721. doi: 10.1007/s11033-022-08193-4. Epub 2022 Dec 23. Mol Biol Rep. 2023. PMID: 36562937 Free PMC article. Review.
-
Inhibition of S-protein RBD and hACE2 Interaction for Control of SARSCoV- 2 Infection (COVID-19).Mini Rev Med Chem. 2021;21(6):689-703. doi: 10.2174/1389557520666201117111259. Mini Rev Med Chem. 2021. PMID: 33208074 Review.
Cited by
-
Design of a bifunctional pan-sarbecovirus entry inhibitor targeting the cell receptor and viral fusion protein.J Virol. 2023 Aug 31;97(8):e0019223. doi: 10.1128/jvi.00192-23. Epub 2023 Aug 14. J Virol. 2023. PMID: 37578234 Free PMC article.
-
Efficient Expression in Leishmania tarentolae (LEXSY) of the Receptor-Binding Domain of the SARS-CoV-2 S-Protein and the Acetylcholine-Binding Protein from Lymnaea stagnalis.Molecules. 2024 Feb 21;29(5):943. doi: 10.3390/molecules29050943. Molecules. 2024. PMID: 38474455 Free PMC article.
-
Allosteric Signal within the Receptor-Binding Domain of the SARS-CoV-2 Spike Protein Mediated by a Class 3 Monoclonal Antibody Revealed through Molecular Dynamics Simulations and Protein Residue Networks.ACS Omega. 2024 Jan 18;9(4):4684-4694. doi: 10.1021/acsomega.3c07947. eCollection 2024 Jan 30. ACS Omega. 2024. PMID: 38313482 Free PMC article.
-
Omicron Coronavirus: pH-Dependent Electrostatic Potential and Energy of Association of Spike Protein to ACE2 Receptor.Viruses. 2023 Aug 17;15(8):1752. doi: 10.3390/v15081752. Viruses. 2023. PMID: 37632094 Free PMC article.
-
Development and characterization of a multimeric recombinant protein using the spike protein receptor binding domain as an antigen to induce SARS-CoV-2 neutralization.Immun Inflamm Dis. 2024 Jul;12(7):e1353. doi: 10.1002/iid3.1353. Immun Inflamm Dis. 2024. PMID: 39056544 Free PMC article.
References
-
- Berguer P.M., Blaustein M., Bredeston L., Craig P.O., D'Alessio C., Elias F., Farré P.C., Fernández N.B., Gentili H.G., Gándola Y., Gasulla, J., Gudesblat, G.E., Herrera, M.G., Ibañez, L.I., Idrovo-Hidalgo, T., Nadra, A.D., Noseda, D.G., Paván, C.H., Pavan, M.F., Pignataro, M.F., Roman, E., Ruberto, L.A.M., Rubinstein, N., Sanchez, M. V, Santos, J., Wetzler, D.E., Zelada, A.M. Production of a highly immunogenic antigen from SARS-CoV-2 by covalent coupling of the receptor binding domain of spike protein to a multimeric carrier. bioRxiv. 2021 doi: 10.1101/2021.04.25.441271. Preprint at. - DOI
-
- Bruchez A., Sha K., Johnson J., Chen L., Stefani C., McConnell H., Gaucherand L., Prins R., Matreyek K.A., Hume A.J., et al. MHC class II transactivator CIITA induces cell resistance to ebola virus and SARS-like coronaviruses. Science. 2020;370:241–247. doi: 10.1126/science.abb3753. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

