The Relative and Combined Effects of Noise Exposure and Aging on Auditory Peripheral Neural Deafferentation: A Narrative Review
- PMID: 35813954
- PMCID: PMC9260498
- DOI: 10.3389/fnagi.2022.877588
The Relative and Combined Effects of Noise Exposure and Aging on Auditory Peripheral Neural Deafferentation: A Narrative Review
Abstract
Animal studies have shown that noise exposure and aging cause a reduction in the number of synapses between low and medium spontaneous rate auditory nerve fibers and inner hair cells before outer hair cell deterioration. This noise-induced and age-related cochlear synaptopathy (CS) is hypothesized to compromise speech recognition at moderate-to-high suprathreshold levels in humans. This paper evaluates the evidence on the relative and combined effects of noise exposure and aging on CS, in both animals and humans, using histopathological and proxy measures. In animal studies, noise exposure seems to result in a higher proportion of CS (up to 70% synapse loss) compared to aging (up to 48% synapse loss). Following noise exposure, older animals, depending on their species, seem to either exhibit significant or little further synapse loss compared to their younger counterparts. In humans, temporal bone studies suggest a possible age- and noise-related auditory nerve fiber loss. Based on the animal data obtained from different species, we predict that noise exposure may accelerate age-related CS to at least some extent in humans. In animals, noise-induced and age-related CS in separation have been consistently associated with a decreased amplitude of wave 1 of the auditory brainstem response, reduced middle ear muscle reflex strength, and degraded temporal processing as demonstrated by lower amplitudes of the envelope following response. In humans, the individual effects of noise exposure and aging do not seem to translate clearly into deficits in electrophysiological, middle ear muscle reflex, and behavioral measures of CS. Moreover, the evidence on the combined effects of noise exposure and aging on peripheral neural deafferentation in humans using electrophysiological and behavioral measures is even more sparse and inconclusive. Further research is necessary to establish the individual and combined effects of CS in humans using temporal bone, objective, and behavioral measures.
Keywords: age-related hearing loss (ARHL); auditory brainstem response (ABR); cochlear synaptopathy (CS); envelope-following response (EFR); middle ear muscle reflex (MEMR); noise exposure; speech-perception-in-noise (SPiN); summating potential to action potential ratio (SP:AP).
Copyright © 2022 Shehabi, Prendergast and Plack.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


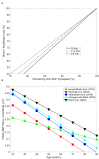
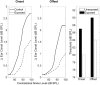
References
-
- Altschuler R. A., Dolan D. F., Halsey K., Kanicki A., Deng N., Martin C., et al. . (2015). Age-related changes in auditory nerve-inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice. Neuroscience 292, 22–33. 10.1016/j.neuroscience.2015.01.068 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

