Role of Phosphorus-Containing Molecules on the Formation of Nano-Sized Calcium Phosphate for Bone Therapy
- PMID: 35813995
- PMCID: PMC9257216
- DOI: 10.3389/fbioe.2022.875531
Role of Phosphorus-Containing Molecules on the Formation of Nano-Sized Calcium Phosphate for Bone Therapy
Abstract
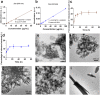
Calcium phosphate (CaP) is the principal inorganic constituent of bone and teeth in vertebrates and has various applications in biomedical areas. Among various types of CaPs, amorphous calcium phosphate (ACP) is considered to have superior bioactivity and biodegradability. With regard to the instability of ACP, the phosphorus-containing molecules are usually adopted to solve this issue, but the specific roles of the molecules in the formation of nano-sized CaP have not been clearly clarified yet. Herein, alendronate, cyclophosphamide, zoledronate, and foscarnet are selected as the model molecules, and theoretical calculations were performed to elucidate the interaction between calcium ions and different model molecules. Subsequently, CaPs were prepared with the addition of the phosphorus-containing molecules. It is found that cyclophosphamide has limited influence on the generation of CaPs due to their weak interaction. During the co-precipitation process of Ca2+ and PO4 3-, the competitive relation among alendronate, zoledronate, and foscarnet plays critical roles in the produced inorganic-organic complex. Moreover, the biocompatibility of CaPs was also systematically evaluated. The DFT calculation provides a convincing strategy for predicting the structure of CaPs with various additives. This work is promising for designing CaP-based multifunctional drug delivery systems and tissue engineering materials.
Keywords: bone therapy; calcium phosphate; drug delivery; nanocomposites; phosphorus-containing molecules.
Copyright © 2022 Jiang, Tao, Chen, Xue, Ding, Wang, Liu, Li and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




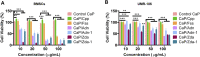
Similar articles
-
Utility of Amorphous Calcium Phosphate-Based Scaffolds in Dental/Biomedical Applications.Biointerface Res Appl Chem. 2017;7(1):1989-1994. Epub 2017 Feb 15. Biointerface Res Appl Chem. 2017. PMID: 29225960 Free PMC article.
-
Advances in synthesis of calcium phosphate crystals with controlled size and shape.Acta Biomater. 2014 Oct;10(10):4071-102. doi: 10.1016/j.actbio.2014.06.017. Epub 2014 Jun 20. Acta Biomater. 2014. PMID: 24954909 Review.
-
Preparation of porous calcium phosphate microspheres with phosphate-containing molecules at room temperature for drug delivery and osteogenic differentiation.RSC Adv. 2018 Jul 16;8(45):25480-25488. doi: 10.1039/c8ra03943g. eCollection 2018 Jul 16. RSC Adv. 2018. PMID: 35539788 Free PMC article.
-
Strontium-Doped Amorphous Calcium Phosphate Porous Microspheres Synthesized through a Microwave-Hydrothermal Method Using Fructose 1,6-Bisphosphate as an Organic Phosphorus Source: Application in Drug Delivery and Enhanced Bone Regeneration.ACS Appl Mater Interfaces. 2017 Feb 1;9(4):3306-3317. doi: 10.1021/acsami.6b12325. Epub 2017 Jan 24. ACS Appl Mater Interfaces. 2017. PMID: 28068758
-
Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications.Chem Soc Rev. 2019 May 20;48(10):2698-2737. doi: 10.1039/c8cs00489g. Chem Soc Rev. 2019. PMID: 31080987 Review.
Cited by
-
Targeted and responsive biomaterials in osteoarthritis.Theranostics. 2023 Jan 16;13(3):931-954. doi: 10.7150/thno.78639. eCollection 2023. Theranostics. 2023. PMID: 36793867 Free PMC article. Review.
References
-
- Ding H., Pan H., Xu X., Tang R. (2014). Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth & Des. 14 (2), 763–769. 10.1021/cg401619s - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

