Low-Stiffness Hydrogels Promote Peripheral Nerve Regeneration Through the Rapid Release of Exosomes
- PMID: 35814007
- PMCID: PMC9260118
- DOI: 10.3389/fbioe.2022.922570
Low-Stiffness Hydrogels Promote Peripheral Nerve Regeneration Through the Rapid Release of Exosomes
Abstract
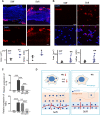
A hydrogel system loaded with mesenchymal stem cell-derived exosome (MSC-Exos) is an attractive new tool for tissue regeneration. However, the effect of the stiffness of exosome-loaded hydrogels on tissue regeneration is unclear. Here, the role of exosome-loaded hydrogel stiffness, during the regeneration of injured nerves, was assessed in vivo. The results showed that the photocrosslinkable hyaluronic acid methacrylate hydrogel stiffness plays an important role in repairing nerve injury. Compared with the stiff hydrogels loaded with exosomes, soft hydrogels loaded with exosomes showed better repair of injured peripheral nerves. The soft hydrogel promoted nerve repair by quickly releasing exosomes to inhibit the infiltration of macrophages and the expression of the proinflammatory factors IL-1β and TNF-α in injured nerves. Our work revealed that exosome-loaded hydrogel stiffness plays an important role in tissue regeneration by regulating exosome release behavior and provided important clues for the clinical application of biological scaffold materials.
Keywords: exosome release behavior; hydrogel stiffness; mesenchymal stem cell–derived exosomes; nerve injury inflammation; sciatic nerve injury.
Copyright © 2022 Liu, Tong, Li, Wang, Fan, Song, Yang, Wang, Jiang, Zhou, Yuan and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Chen T., Li Y., Ni W., Tang B., Wei Y., Li J., et al. (2020). Human Neural Stem Cell-Conditioned Medium Inhibits Inflammation in Macrophages via Sirt-1 Signaling Pathway In Vitro and Promotes Sciatic Nerve Injury Recovery in Rats. Stem Cells Dev. 29 (16), 1084–1095. 10.1089/scd.2020.0020 - DOI - PubMed
LinkOut - more resources
Full Text Sources

