High-Frequency 10-kHz Spinal Cord Stimulation Improves Health-Related Quality of Life in Patients With Refractory Painful Diabetic Neuropathy: 12-Month Results From a Randomized Controlled Trial
- PMID: 35814185
- PMCID: PMC9256824
- DOI: 10.1016/j.mayocpiqo.2022.05.003
High-Frequency 10-kHz Spinal Cord Stimulation Improves Health-Related Quality of Life in Patients With Refractory Painful Diabetic Neuropathy: 12-Month Results From a Randomized Controlled Trial
Abstract
Objective: To evaluate high-frequency (10-kHz) spinal cord stimulation (SCS) treatment in refractory painful diabetic neuropathy.
Patients and methods: A prospective, multicenter randomized controlled trial was conducted between Aug 28, 2017 and March 16, 2021, comparing conventional medical management (CMM) with 10-kHz SCS+CMM. The participants had hemoglobin A1c level of less than or equal to 10% and pain greater than or equal to 5 of 10 cm on visual analog scale, with painful diabetic neuropathy symptoms 12 months or more refractory to gabapentinoids and at least 1 other analgesic class. Assessments included measures of pain, neurologic function, and health-related quality of life (HRQoL) over 12 months with optional crossover at 6 months.
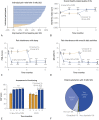
Results: The participants were randomized 1:1 to CMM (n=103) or 10-kHz SCS+CMM (n=113). At 6 months, 77 of 95 (81%) CMM group participants opted for crossover, whereas none of the 10-kHz SCS group participants did so. At 12 months, the mean pain relief from baseline among participants implanted with 10-kHz SCS was 74.3% (95% CI, 70.1-78.5), and 121 of 142 (85%) participants were treatment responders (≥50% pain relief). Treatment with 10-kHz SCS improved HRQoL, including a mean improvement in the EuroQol 5-dimensional questionnaire index score of 0.136 (95% CI, 0.104-0.169). The participants also reported significantly less pain interference with sleep, mood, and daily activities. At 12 months, 131 of 142 (92%) participants were "satisfied" or "very satisfied" with the 10-kHz SCS treatment.
Conclusion: The 10-kHz SCS treatment resulted in substantial pain relief and improvement in overall HRQoL 2.5- to 4.5-fold higher than the minimal clinically important difference. The outcomes were durable over 12 months and support 10-kHz SCS treatment in patients with refractory painful diabetic neuropathy.
Trial registration: clincaltrials.gov Identifier: NCT03228420.
Keywords: CMM, conventional medical management; DN4, Douleur Neuropathique; DSPN, diabetic sensorimotor peripheral neuropathy; EQ-5D-5L, EuroQol 5-Dimension 5-Level questionnaire; HRQoL, health-related quality of life; HbA1c, hemoglobin A1c; IPG, implantable pulse generator; NNT, number needed to treat; PDN, painful diabetic neuropathy; RCT, randomized controlled trial; SCS, spinal cord stimulation; VAS, visual analog scale.
© 2022 The Authors.
Figures
Similar articles
-
Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: A Randomized Clinical Trial.JAMA Neurol. 2021 Jun 1;78(6):687-698. doi: 10.1001/jamaneurol.2021.0538. JAMA Neurol. 2021. PMID: 33818600 Free PMC article. Clinical Trial.
-
Long-term efficacy of high-frequency (10 kHz) spinal cord stimulation for the treatment of painful diabetic neuropathy: 24-Month results of a randomized controlled trial.Diabetes Res Clin Pract. 2023 Sep;203:110865. doi: 10.1016/j.diabres.2023.110865. Epub 2023 Aug 1. Diabetes Res Clin Pract. 2023. PMID: 37536514 Free PMC article. Clinical Trial.
-
High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: design of a multicenter, randomized controlled trial (SENZA-PDN).Trials. 2020 Jan 15;21(1):87. doi: 10.1186/s13063-019-4007-y. Trials. 2020. PMID: 31941531 Free PMC article. Clinical Trial.
-
Indirect Comparison of 10 kHz Spinal Cord Stimulation (SCS) versus Traditional Low-Frequency SCS for the Treatment of Painful Diabetic Neuropathy: A Systematic Review of Randomized Controlled Trials.Biomedicines. 2022 Oct 19;10(10):2630. doi: 10.3390/biomedicines10102630. Biomedicines. 2022. PMID: 36289892 Free PMC article. Review.
-
Spinal cord stimulation in painful diabetic neuropathy: An overview.Diabetes Res Clin Pract. 2023 Dec;206 Suppl 1:110760. doi: 10.1016/j.diabres.2023.110760. Diabetes Res Clin Pract. 2023. PMID: 38245324 Review.
Cited by
-
Spinal Cord Stimulation for Painful Diabetic Neuropathy.J Diabetes Sci Technol. 2024 Jan;18(1):168-192. doi: 10.1177/19322968221133795. Epub 2022 Nov 17. J Diabetes Sci Technol. 2024. PMID: 36384312 Free PMC article. Review.
-
Screening for diabetic peripheral neuropathy in resource-limited settings.Diabetol Metab Syndr. 2023 Mar 22;15(1):55. doi: 10.1186/s13098-023-01032-x. Diabetol Metab Syndr. 2023. PMID: 36945043 Free PMC article. Review.
-
A Systematic Guideline by the ASPN Workgroup on the Evidence, Education, and Treatment Algorithm for Painful Diabetic Neuropathy: SWEET.J Pain Res. 2024 Apr 13;17:1461-1501. doi: 10.2147/JPR.S451006. eCollection 2024. J Pain Res. 2024. PMID: 38633823 Free PMC article. Review.
-
Long-term efficacy of 10 kHz spinal cord stimulation in managing painful diabetic neuropathy: A post-study survey.Pain Pract. 2025 Jun;25(5):e70023. doi: 10.1111/papr.70023. Pain Pract. 2025. PMID: 40242901 Free PMC article. Clinical Trial.
-
Post-trial access to implantable neural devices: an exploratory international survey.BMJ Surg Interv Health Technol. 2024 Apr 17;6(1):e000262. doi: 10.1136/bmjsit-2024-000262. eCollection 2024. BMJ Surg Interv Health Technol. 2024. PMID: 38646454 Free PMC article.
References
-
- Global report on diabetes. World Health Organization. 2016. https://www.who.int/publications/i/item/9789241565257
-
- Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157 - PubMed
-
- Sloan G., Selvarajah D., Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17(7):400–420. - PubMed
-
- Boulton A.J., Armstrong D.G., Albert S.F., et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–1685. - PMC - PubMed
-
- Rastogi A., Goyal G., Kesavan R., et al. Long term outcomes after incident diabetic foot ulcer: multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study: epidemiology of diabetic foot complications study. Diabetes Res Clin Pract. 2020;162 - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

