Profiling Differential Effects of 5 Selective Serotonin Reuptake Inhibitors on TLRs-Dependent and -Independent IL-6 Production in Immune Cells Identifies Fluoxetine as Preferred Anti-Inflammatory Drug Candidate
- PMID: 35814203
- PMCID: PMC9257214
- DOI: 10.3389/fphar.2022.874375
Profiling Differential Effects of 5 Selective Serotonin Reuptake Inhibitors on TLRs-Dependent and -Independent IL-6 Production in Immune Cells Identifies Fluoxetine as Preferred Anti-Inflammatory Drug Candidate
Abstract
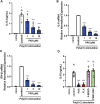
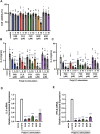
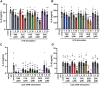
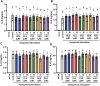
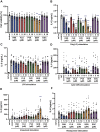
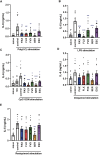
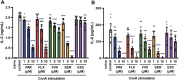
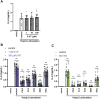
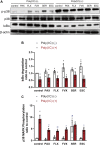
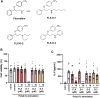
Excessive proinflammatory cytokine production induced by abnormal activation of Toll-like receptor (TLR) signaling, for example, by SARS-CoV-2 infection, can cause a fatal cytokine storm. The selective serotonin reuptake inhibitors (SSRIs) fluoxetine and fluvoxamine, used to treat depression, were recently reported to reduce the risk of severe disease in patients with coronavirus disease 2019 (COVID-19), but the mechanisms of the anti-inflammatory effects of SSRIs, and which SSRI would be most suitable as an anti-inflammatory drug, remain unclear. Here, we examined the inhibitory effects of 5 FDA-approved SSRIs, paroxetine, fluoxetine, fluvoxamine, sertraline and escitalopram, on the production of interleukin-6 (IL-6) induced by stimulation with multiple TLR agonists in murine macrophages and dendritic cells, and on the production of cytokines induced by concanavalin A in murine lymphocytes. In J774.1 murine macrophage cells, pretreatment with SSRIs significantly suppressed IL-6 release induced by TLR3 agonist poly(I:C), TLR4 agonist LPS or TLR9 agonist CpG ODN, but did not affect IL-6 release induced by TLR7 agonists imiquimod or resiquimod. In accordance with the results obtained in J774.1 cells, pretreatment with SSRIs also suppressed IL-6 release induced by a TLR3, TLR4 or TLR9 agonist in bone marrow-derived dendritic cells and peritoneal cells of C57BL/6 mice. On the other hand, interestingly, sertraline alone among the SSRIs amplified IL-6 production induced by TLR7 agonists in murine dendritic cells, though not in macrophages. Concanavalin A-induced production of IL-6 or IL-2 in murine lymphocytes was suppressed by SSRIs, suggesting that SSRIs also inhibit TLRs-independent IL-6 production. Since SSRIs suppressed both IL-6 production induced by multiple TLR agonists in macrophages or dendritic cells and TLR-independent IL-6 production in lymphocytes, they are promising candidates for treatment of patients with cytokine storm, which is mediated by overactivation of multiple TLRs in a complex manner, leading to the so-called IL-6 amplifier, an IL-6 overproduction loop. However, the 5 SSRIs examined here all showed different effects. Overall, our results suggest that fluoxetine may be the most promising candidate as an anti-inflammatory drug. An examination of the structural requirements indicated that the N-methyl group of fluoxetine has a critical role in the inhibition of IL-6 production.
Keywords: IL-6; Toll-like receptor; concanavalin A; cytokine; dendritic cells; lymphocytes; macrophage; selective serotonin reuptake inhibitor.
Copyright © 2022 Takenaka, Tanaka, Kitabatake, Kuramochi, Aoki and Tsukimoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Structure-Activity Relationship Analysis of Fluoxetine for Suppression of Inflammatory Cytokine Production.Biol Pharm Bull. 2024;47(5):946-954. doi: 10.1248/bpb.b24-00083. Biol Pharm Bull. 2024. PMID: 38735732
-
Selective serotonin reuptake inhibitor attenuates the hyperresponsiveness of TLR2+ and TLR4+ Th17/Tc17-like cells in multiple sclerosis patients with major depression.Immunology. 2021 Mar;162(3):290-305. doi: 10.1111/imm.13281. Epub 2020 Nov 23. Immunology. 2021. PMID: 33112414 Free PMC article.
-
Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors.Arthritis Rheum. 2010 Mar;62(3):683-93. doi: 10.1002/art.27304. Arthritis Rheum. 2010. PMID: 20131240
-
Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review.Neurol Int. 2021 Aug 5;13(3):387-401. doi: 10.3390/neurolint13030038. Neurol Int. 2021. PMID: 34449705 Free PMC article. Review.
-
Selective serotonin reuptake inhibitors: New hope in the fight against COVID-19.Front Pharmacol. 2022 Nov 30;13:1036093. doi: 10.3389/fphar.2022.1036093. eCollection 2022. Front Pharmacol. 2022. PMID: 36532776 Free PMC article. Review.
Cited by
-
Fluvoxamine for the treatment of COVID-19.Cochrane Database Syst Rev. 2022 Sep 14;9(9):CD015391. doi: 10.1002/14651858.CD015391. Cochrane Database Syst Rev. 2022. PMID: 36103313 Free PMC article.
-
The IRE1α-XBP1 arm of the unfolded protein response is a host factor activated in SARS-CoV-2 infection.Biochim Biophys Acta Mol Basis Dis. 2024 Jun;1870(5):167193. doi: 10.1016/j.bbadis.2024.167193. Epub 2024 Apr 20. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38648902 Free PMC article.
-
Immuno-oncological effects of standard anticancer agents and commonly used concomitant drugs: an in vitro assessment.BMC Pharmacol Toxicol. 2024 Mar 5;25(1):25. doi: 10.1186/s40360-024-00746-6. BMC Pharmacol Toxicol. 2024. PMID: 38444002 Free PMC article.
-
Host 5-HT affects Plasmodium transmission in mosquitoes via modulating mosquito mitochondrial homeostasis.PLoS Pathog. 2024 Oct 15;20(10):e1012638. doi: 10.1371/journal.ppat.1012638. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39405338 Free PMC article.
-
Paroxetine attenuated liver fibrosis in liver of bile duct ligated rats: role of P2X4 purinoreceptor.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 23. doi: 10.1007/s00210-025-04278-6. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40407866
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

