Genetic Polymorphisms in CYP2C19 Cause Changes in Plasma Levels and Adverse Reactions to Anlotinib in Chinese Patients With Lung Cancer
- PMID: 35814206
- PMCID: PMC9257029
- DOI: 10.3389/fphar.2022.918219
Genetic Polymorphisms in CYP2C19 Cause Changes in Plasma Levels and Adverse Reactions to Anlotinib in Chinese Patients With Lung Cancer
Abstract
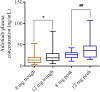
Background: Anlotinib is a small molecular multi-targeting tyrosine kinase inhibitor. Growing evidence indicates that treatment efficacy, and toxicity varies considerably between individuals. Therefore, this study aimed to investigate the relationship between cytochrome P450 (CYP450) gene polymorphisms, drug concentrations, and their adverse reactions in anlotinib-treated patients with lung cancer. Methods: We enrolled 139 patients with lung cancer, treated with anlotinib. Twenty loci in the following five genes of the CYP450 family were genotyped: CYP450 family 3 subfamily A member 5 (CYP3A5), 3 subfamily A member 4 (CYP3A4), 2 subfamily C member 9 (CYP2C9), 2 subfamily C member 19 (CYP2C19), and 1 subfamily A member 2 (CYP1A2). Data on adverse reactions were collected from patients, and plasma anlotinib concentrations were measured. Results: There were significant variances in plasma trough concentration (3.95-52.88 ng/ml) and peak plasma concentration (11.53-42.8 ng/ml) following administration of 8 mg anlotinib. Additionally, there were significant differences in the plasma trough concentration (5.65-81.89 ng/ml) and peak plasma concentration (18.01-107.18 ng/ml) following administration of 12 mg anlotinib. Furthermore, for CYP2C19-rs3814637, the peak plasma concentrations of mutant allele T carriers (TT+CT) were significantly higher than those of wildtypes (CC). For CYP2C19-rs11568732, the peak plasma concentrations of the mutant allele G carriers (GT+GG) were significantly higher than those of the wild-type (TT). More importantly, the incidence rates of hypertension and hemoptysis (peripheral lung cancer) with TT+CT in rs3814637 and GT+GG in rs11568732 were significantly higher than those with CC and TT. Conclusions: The plasma trough and peak concentrations varied significantly for both 8 and 12 mg of anlotinib. Single-nucleotide polymorphisms in CYP2C19 are significantly associated with hypertension, hemoptysis, and anlotinib peak concentrations. Polymorphisms in CYP450 may explain inter-individual differences in anlotinib-related adverse reactions.
Keywords: CYP450 gene polymorphisms; adverse reactions; anlotinib; lung cancer; plasma concentration.
Copyright © 2022 Tan, Han, Cheng, Jiang, Zhang, Xia, Wang and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Polymorphisms of Drug-Metabolizing Enzymes and Transporters Contribute to the Individual Variations of Erlotinib Steady State Trough Concentration, Treatment Outcomes, and Adverse Reactions in Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer Patients.Front Pharmacol. 2020 May 8;11:664. doi: 10.3389/fphar.2020.00664. eCollection 2020. Front Pharmacol. 2020. PMID: 32457635 Free PMC article.
-
Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment.Clin Pharmacol Ther. 2005 Dec;78(6):593-604. doi: 10.1016/j.clpt.2005.08.011. Clin Pharmacol Ther. 2005. PMID: 16338275 Clinical Trial.
-
The impact of cytochrome P450 3A genetic polymorphisms on tacrolimus pharmacokinetics in ulcerative colitis patients.PLoS One. 2021 Apr 22;16(4):e0250597. doi: 10.1371/journal.pone.0250597. eCollection 2021. PLoS One. 2021. PMID: 33886687 Free PMC article.
-
China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy.Cancer Commun (Lond). 2019 Jun 20;39(1):36. doi: 10.1186/s40880-019-0383-7. Cancer Commun (Lond). 2019. PMID: 31221221 Free PMC article. Review.
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I.Clin Pharmacokinet. 2010 Mar;49(3):141-75. doi: 10.2165/11317350-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20170205 Review.
Cited by
-
Prediction of Pharmacokinetic Drug-Drug Interactions Involving Anlotinib as a Victim by Using Physiologically Based Pharmacokinetic Modeling.Drug Des Devel Ther. 2024 Oct 15;18:4585-4600. doi: 10.2147/DDDT.S480402. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39429896 Free PMC article.
-
The Effect of CYP2C19*2 (rs4244285) and CYP17 (rs743572) SNPs on Adriamycin and Paclitaxel based Chemotherapy Outcomes in Breast Cancer Patients.Asian Pac J Cancer Prev. 2024 Jun 1;25(6):1977-1986. doi: 10.31557/APJCP.2024.25.6.1977. Asian Pac J Cancer Prev. 2024. PMID: 38918659 Free PMC article.
-
Association between anlotinib trough plasma concentration and treatment outcomes in advanced non-small-cell lung cancer.Front Oncol. 2023 Mar 3;13:1146362. doi: 10.3389/fonc.2023.1146362. eCollection 2023. Front Oncol. 2023. PMID: 36937430 Free PMC article.
-
Construction of HBV-HCC prognostic model and immune characteristics based on potential genes mining through protein interaction networks.J Cancer Res Clin Oncol. 2023 Oct;149(13):11263-11278. doi: 10.1007/s00432-023-04989-4. Epub 2023 Jun 26. J Cancer Res Clin Oncol. 2023. PMID: 37358667 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous