Metformin With or Without Clomiphene Citrate Versus Laparoscopic Ovarian Drilling With or Without Clomiphene Citrate to Treat Patients With Clomiphene Citrate-Resistant Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis
- PMID: 35814214
- PMCID: PMC9256960
- DOI: 10.3389/fphar.2022.576458
Metformin With or Without Clomiphene Citrate Versus Laparoscopic Ovarian Drilling With or Without Clomiphene Citrate to Treat Patients With Clomiphene Citrate-Resistant Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis
Abstract
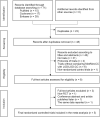
Introduction: Which is optimal to treat clomiphene citrate-resistant polycystic ovary syndrome (CCR-PCOS) with LOD or metformin remains a problem. There are three inconsistent or even contradictory views. Objectives: The present meta-analysis aimed to evaluate the effectiveness and safety of Metformin with or without CC and to compare them with LOD with or without CC (Met/Met-CC vs. LOD/LOD-CC) in women with CCR-PCOS who also have anovulation. Data source: The PubMed, Cochrane, and Embase databases were searched to identify relevant studies reported between 1 Jan 1966 and 31 Aug 2019; the search was updated on 17 May 2022. Study eligibility criteria: We included randomized controlled trials (RCTs) of CCR-PCOS that had considered Met/Met-CC and LOD/LOD-CC as the exposure variables and fertility as the main outcome variable. Study appraisal and synthesis methods: We assessed study quality using the Cochrane risk-of-bias tool. The primary effectiveness outcome was live birth/ongoing pregnancy rate and the primary safety outcome was miscarriage rate. A fixed-effect meta-analysis was performed. The robustness of the results was assessed using sensitivity analyses. Meta-regression and subgroup analysis were performed to examine the reasons for heterogeneity. Publication bias was examined using the funnel plot, Egger linear regression, and Begg rank correlation tests. The quality of this meta-analysis was estimated according to the GRADE approach. This meta-analysis has been registered in PROSPERO (CRD42021240156). Results: Among 71 potentially relevant studies, we included five RCTs in our meta-analysis. We found no difference in effectiveness between Met-CC and LOD in terms of live birth/ongoing pregnancy (RR = 1.02, 95% CI: 0.87-1.21, z = 0.28; p = 0.780), and miscarriage rates (RR = 0.79, 95% CI: 0.46-1.36, z = 0.86; p = 0.390). I2 tests results revealed moderate or no heterogeneity (I2 = 51.4%, p = 0.083; I2= 0.0%; p = 0.952). Sensitivity analysis confirmed the robustness of the results. Funnel plot, Egger linear regression, and Begg rank correlation tests implied no publication bias (p > 0.05). LOD was more expensive than Met (€1050 vs. €50.16). The evidence quality was moderate. Conclusion: There is no evidence on the difference in the outcomes between the two interventions regarding ovulation, pregnancy, and live birth. As LOD is an invasive procedure and carries inherent risks, the use of Met/Met-CC should be the second-line treatment for women with CCR-PCOS. Systematic Review Registration: identifier CRD42021240156.
Keywords: clomiphene citrate resistance; laparoscopic ovarian drilling; live-birth; meta-analysis; metformin.
Copyright © 2022 Sun, Bai, Song, Wang, Gao, Zheng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome.Cochrane Database Syst Rev. 2020 Feb 11;2(2):CD001122. doi: 10.1002/14651858.CD001122.pub5. Cochrane Database Syst Rev. 2020. PMID: 32048270 Free PMC article.
-
First-line ovulation induction for polycystic ovary syndrome: an individual participant data meta-analysis.Hum Reprod Update. 2019 Nov 5;25(6):717-732. doi: 10.1093/humupd/dmz029. Hum Reprod Update. 2019. PMID: 31647106
-
Ultrasound-guided transvaginal ovarian needle drilling for clomiphene-resistant polycystic ovarian syndrome in subfertile women.Cochrane Database Syst Rev. 2019 Jul 31;7(7):CD008583. doi: 10.1002/14651858.CD008583.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 4;11:CD008583. doi: 10.1002/14651858.CD008583.pub3. PMID: 31425630 Free PMC article. Updated. Review.
-
Letrozole versus laparoscopic ovarian drilling in clomiphene citrate-resistant women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials.Reprod Biol Endocrinol. 2019 Feb 6;17(1):17. doi: 10.1186/s12958-019-0461-3. Reprod Biol Endocrinol. 2019. PMID: 30728032 Free PMC article.
-
Laparoscopic ovarian diathermy vs clomiphene citrate plus metformin as second-line strategy for infertile anovulatory patients with polycystic ovary syndrome: a randomized controlled trial.Am J Obstet Gynecol. 2010 Jun;202(6):577.e1-8. doi: 10.1016/j.ajog.2009.11.042. Epub 2010 Jan 22. Am J Obstet Gynecol. 2010. PMID: 20096821 Clinical Trial.
Cited by
-
Transvaginal ovarian drilling for polycystic ovary syndrome prior to in vitro fertilization dramatically improves embryo yield, implantation, and ongoing pregnancy rates.J Assist Reprod Genet. 2025 Mar;42(3):839-845. doi: 10.1007/s10815-024-03362-9. Epub 2024 Dec 24. J Assist Reprod Genet. 2025. PMID: 39718714
References
-
- Abu Hashim H., El Lakany N., Sherief L. (2011). Combined Metformin and Clomiphene Citrate versus Laparoscopic Ovarian Diathermy for Ovulation Induction in Clomiphene-Resistant Women with Polycystic Ovary Syndrome: a Randomized Controlled Trial. J. Obstet. Gynaecol. Res. 37 (3), 169–177. 10.1111/j.1447-0756.2010.01383.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

