Extra Spindle Pole Bodies-Like 1 Serves as a Prognostic Biomarker and Promotes Lung Adenocarcinoma Metastasis
- PMID: 35814478
- PMCID: PMC9257280
- DOI: 10.3389/fonc.2022.930647
Extra Spindle Pole Bodies-Like 1 Serves as a Prognostic Biomarker and Promotes Lung Adenocarcinoma Metastasis
Abstract
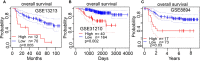
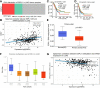
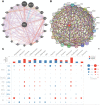
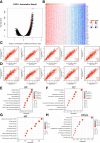
Extra spindle pole bodies-like 1 (ESPL1), a cysteine endopeptidase, plays a vital role in chromosome inheritance. However, the association of ESPL1 with prognosis and immune infiltration in lung adenocarcinoma (LUAD) has not yet been explored. Here, we analyzed the expression level, prognostic values, diagnostic value, and immune infiltration level in LUAD using various databases. Immunohistochemistry (IHC) and quantitative real-time PCR (qRT-PCR) assays were used to detect the expression of ESPL1 in LUAD tissues and cell lines. In this study, we found that ESPL1 was upregulated in LUAD and a higher expression of ESPL1 was correlated with unfavorable prognosis in LUAD. Meanwhile, Cox hazard regression analysis results suggested that ESPL1 may be an independent prognostic factor for LUAD. Moreover, we demonstrated that ESPL1 expression was significantly correlated with immune infiltration of Th2 and dendritic cells in LUAD. We also confirmed that DNA copy number amplification and DNA hypo-methylation were positively correlated with ESPL1 expression in LUAD. Additionally, DNA copy number amplification was significantly associated with adverse clinical outcomes in LUAD. Kyoto Encyclopedia of Genes and Genomes (KEGG) and gene set enrichment analysis (GSEA) confirmed that ESPL1 was mainly involved in the DNA replication and glycolysis signaling pathway. Finally, we revealed that ESPL1 was highly expressed in LUAD tissues and cell lines. Knockdown of ESPL1 significantly inhibited cell migration and the invasion abilities of LUAD. Our study comprehensively confirmed that ESPL1 expression may serve as a novel prognostic biomarker for both the clinical outcome and immune cell infiltration in LUAD.
Keywords: DNA methylation; extra spindle pole bodies-like 1; immune infiltration; lung adenocarcinoma; prognosis biomarker.
Copyright © 2022 Nie, Pu, Han, Wang, Pan, Li, Ma, Yao, Zhao, Wang, Jiang and Ding.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
ESPL1 is Elevated in Hepatocellular Carcinoma and Predicts Prognosis.Int J Gen Med. 2022 Nov 28;15:8381-8398. doi: 10.2147/IJGM.S381188. eCollection 2022. Int J Gen Med. 2022. PMID: 36465268 Free PMC article.
-
Integrated analysis and validation reveal ACAP1 as a novel prognostic biomarker associated with tumor immunity in lung adenocarcinoma.Comput Struct Biotechnol J. 2022 Aug 13;20:4390-4401. doi: 10.1016/j.csbj.2022.08.026. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36051873 Free PMC article.
-
AUNIP Expression Is Correlated With Immune Infiltration and Is a Candidate Diagnostic and Prognostic Biomarker for Hepatocellular Carcinoma and Lung Adenocarcinoma.Front Oncol. 2020 Dec 9;10:590006. doi: 10.3389/fonc.2020.590006. eCollection 2020. Front Oncol. 2020. PMID: 33363020 Free PMC article.
-
Upregulation of GNPNAT1 Predicts Poor Prognosis and Correlates With Immune Infiltration in Lung Adenocarcinoma.Front Mol Biosci. 2021 Mar 25;8:605754. doi: 10.3389/fmolb.2021.605754. eCollection 2021. Front Mol Biosci. 2021. PMID: 33842535 Free PMC article.
-
NcRNA-Mediated High Expression of HMMR as a Prognostic Biomarker Correlated With Cell Proliferation and Cell Migration in Lung Adenocarcinoma.Front Oncol. 2022 Mar 3;12:846536. doi: 10.3389/fonc.2022.846536. eCollection 2022. Front Oncol. 2022. PMID: 35311097 Free PMC article.
Cited by
-
Identifying Hub Genes and miRNAs Associated with Alzheimer's Disease: A Bioinformatics Pathway to Novel Therapeutic Strategies.Biomolecules. 2024 Dec 20;14(12):1641. doi: 10.3390/biom14121641. Biomolecules. 2024. PMID: 39766348 Free PMC article.
-
Analysis of Nucleoporin 107 Overexpression and Its Association with Prognosis and Immune Infiltration in Lung Adenocarcinoma by Bioinformatics Methods.Int J Gen Med. 2023 Nov 22;16:5449-5465. doi: 10.2147/IJGM.S441185. eCollection 2023. Int J Gen Med. 2023. PMID: 38021066 Free PMC article.
-
Overexpression of C1orf74 predicts poor outcome and promote cervical cancer progression.Heliyon. 2024 Dec 9;10(24):e40966. doi: 10.1016/j.heliyon.2024.e40966. eCollection 2024 Dec 30. Heliyon. 2024. PMID: 39759315 Free PMC article.
-
The upregulation and transcriptional regulatory mechanisms of Extra spindle pole bodies like 1 in bladder cancer: An immunohistochemistry and high-throughput screening Evaluation.Heliyon. 2024 May 15;10(10):e31192. doi: 10.1016/j.heliyon.2024.e31192. eCollection 2024 May 30. Heliyon. 2024. PMID: 38813236 Free PMC article.
-
ESPL1 is Elevated in Hepatocellular Carcinoma and Predicts Prognosis.Int J Gen Med. 2022 Nov 28;15:8381-8398. doi: 10.2147/IJGM.S381188. eCollection 2022. Int J Gen Med. 2022. PMID: 36465268 Free PMC article.
References
LinkOut - more resources
Full Text Sources

