Saccharomyces Boulardii Ameliorates Non-alcoholic Steatohepatitis in Mice Induced by a Methionine-Choline-Deficient Diet Through Gut-Liver Axis
- PMID: 35814685
- PMCID: PMC9260146
- DOI: 10.3389/fmicb.2022.887728
Saccharomyces Boulardii Ameliorates Non-alcoholic Steatohepatitis in Mice Induced by a Methionine-Choline-Deficient Diet Through Gut-Liver Axis
Abstract
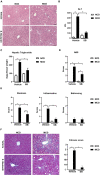
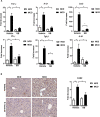
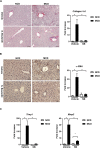
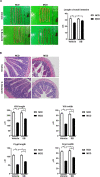
Non-alcoholic steatohepatitis (NASH) is affecting people worldwide. Changes in the intestinal microbiome are crucial to NASH. A previous study showed that eradicating intestinal fungi ameliorates NASH; however, the role of intestinal fungi in the development of NASH remains unclear. Saccharomyces boulardii (SB), a dietary supplement yeast, has been reported to restore the integrity of the intestine. Here, we tested the effect of SB in the treatment of NASH. For this study, we fed eight-week-old C57/BL6 male mice either a methionine-choline deficient (MCD) diet or a normal chow diet (NCD) for eight weeks. Half of the MCD diet-fed mice were gavaged with SB (5 mg/day) once daily. The remainder of the NCD-fed mice were gavaged with normal saline as a control. The MCD diet-fed mice on SB supplement showed better liver function, less hepatic steatosis, and decreased inflammation. Both hepatic inflammatory gene expression and fibrogenic gene expression were suppressed in mice with SB gavage. Intestinal damage caused by the MCD diet was tampered with, intestine inflammation decreased, and gut permeability improved in mice that had been given the SB supplement. Deep sequencing of the fecal microbiome showed a potentially increased beneficial gut microbiota and increased microbiota diversity in the SB-supplemented mice. The SB supplement maintains gut integrity, increases microbial diversity, and increases the number of potentially beneficial gut microbiota. Thus, the SB supplement attenuates gut leakage and exerts a protective effect against NASH. Our results provide new insight into the prevention of NASH.
Keywords: Saccharomyces boulardii; gut-liver axis; intestinal commensal fungi; methionine-choline deficiency diet; non-alcoholic steatohepatitis.
Copyright © 2022 Yang, Lin, Liu, Syu, Sun, Lee, Lin and Hou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Effect of Saccharomyces boulardii on Liver Diseases: A Systematic Review.Microorganisms. 2024 Aug 15;12(8):1678. doi: 10.3390/microorganisms12081678. Microorganisms. 2024. PMID: 39203520 Free PMC article. Review.
-
Intestinal Microbiota Protects against MCD Diet-Induced Steatohepatitis.Int J Mol Sci. 2019 Jan 14;20(2):308. doi: 10.3390/ijms20020308. Int J Mol Sci. 2019. PMID: 30646522 Free PMC article.
-
Soyasaponin A2 Alleviates Steatohepatitis Possibly through Regulating Bile Acids and Gut Microbiota in the Methionine and Choline-Deficient (MCD) Diet-induced Nonalcoholic Steatohepatitis (NASH) Mice.Mol Nutr Food Res. 2021 Jul;65(14):e2100067. doi: 10.1002/mnfr.202100067. Epub 2021 Jun 10. Mol Nutr Food Res. 2021. PMID: 34047448
-
Dynamic alterations in the gut microbiota and metabolome during the development of methionine-choline-deficient diet-induced nonalcoholic steatohepatitis.World J Gastroenterol. 2018 Jun 21;24(23):2468-2481. doi: 10.3748/wjg.v24.i23.2468. World J Gastroenterol. 2018. PMID: 29930468 Free PMC article.
-
A Comparison of the Gene Expression Profiles of Non-Alcoholic Fatty Liver Disease between Animal Models of a High-Fat Diet and Methionine-Choline-Deficient Diet.Molecules. 2022 Jan 27;27(3):858. doi: 10.3390/molecules27030858. Molecules. 2022. PMID: 35164140 Free PMC article. Review.
Cited by
-
Effect of Saccharomyces boulardii on Liver Diseases: A Systematic Review.Microorganisms. 2024 Aug 15;12(8):1678. doi: 10.3390/microorganisms12081678. Microorganisms. 2024. PMID: 39203520 Free PMC article. Review.
-
Gut mycobiome in metabolic diseases: Mechanisms and clinical implication.Biomed J. 2024 Jun;47(3):100625. doi: 10.1016/j.bj.2023.100625. Epub 2023 Jun 25. Biomed J. 2024. PMID: 37364760 Free PMC article. Review.
-
Development and validation of a multimodal model integrating gut microbiota and metabolite for identifying sarcopenia in patients with MASLD: a study from two centers in China.Nutr J. 2025 Aug 22;24(1):129. doi: 10.1186/s12937-025-01198-2. Nutr J. 2025. PMID: 40847308 Free PMC article.
-
Gut microbiota in non-alcoholic fatty liver disease: Pathophysiology, diagnosis, and therapeutics.World J Hepatol. 2025 Jun 27;17(6):106849. doi: 10.4254/wjh.v17.i6.106849. World J Hepatol. 2025. PMID: 40606926 Free PMC article. Review.
-
Microbial underdogs: exploring the significance of low-abundance commensals in host-microbe interactions.Exp Mol Med. 2023 Dec;55(12):2498-2507. doi: 10.1038/s12276-023-01120-y. Epub 2023 Dec 1. Exp Mol Med. 2023. PMID: 38036729 Free PMC article. Review.
References
-
- Brunt E. M., Kleiner D. E., Wilson L. A., Belt P., Neuschwander-Tetri B. A. and NASH Clinical Research Network (CRN) (2011). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 53 810–820. 10.1002/hep.24127 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

