An Exploration of Oral-Gut Pathogens Mediating Immune Escape of Pancreatic Cancer via miR-21/PTEN Axis
- PMID: 35814712
- PMCID: PMC9258743
- DOI: 10.3389/fmicb.2022.928846
An Exploration of Oral-Gut Pathogens Mediating Immune Escape of Pancreatic Cancer via miR-21/PTEN Axis
Abstract
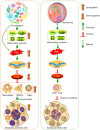
Oral-gut pathogens are closely associated with pancreatic cancer, such as Campylobacter jejuni, Clostridium difficile, Enterococcus faecalis, Escherichia coli, Fusobacterium nucleatum, Helicobacter pylori, Porphyromonas gingivalis, and Vibrio cholera, but the related mechanisms remain not well understood. Phosphatase and tensin homolog (PTEN, a widely known tumor suppressor) play a key role in the anti-cancer immune system. Pancreatic cancer cells with PTEN loss are often in the immunosuppressive tumor microenvironment regulated by myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and M2 macrophages, which are regarded as the mechanism in the immune escape of cancers. The miR-21, as an oncogene in human cancers, plays an important role in pancreatic cancer progression, downregulates the levels of PTEN, and may promote cancer to evade host immune surveillance. Some oral-gut pathogens have been found to promote miR-21 expression and reduce PTEN expression. On the other hand, most gut pathogens infection is thought to produce reactive oxygen species (ROS) or activate inflammatory cytokines, which may also induce ROS-mediated miR-21 expression. These pathogens' infection is involved with the cell density of MDSCs, Tregs, and M2 macrophages. Therefore, it is quite reasonable to propose that oral-gut pathogens possibly promote pancreatic cancer escaping from host immune surveillance by activating the miR-21/PTEN axis and immune-suppressive cells. The present exploration suggests that an increased understanding of the pattern of the effects of gut pathogens on the miR-21/PTEN axis will lead to better insights into the specific mechanisms associated with the immune escape of pancreatic cancer caused by oral-gut microbiota.
Keywords: immune escape; metastasis; miR-21/PTEN axis; oral-gut pathogens; pancreatic cancer.
Copyright © 2022 Li, Hu and Hou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Gum-Gut Axis: Periodontitis and the Risk of Gastrointestinal Cancers.Cancers (Basel). 2023 Sep 15;15(18):4594. doi: 10.3390/cancers15184594. Cancers (Basel). 2023. PMID: 37760563 Free PMC article. Review.
-
Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways.Oncogene. 2018 Aug;37(31):4239-4259. doi: 10.1038/s41388-018-0261-9. Epub 2018 May 1. Oncogene. 2018. PMID: 29713056
-
Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer.World J Gastroenterol. 2016 Jan 14;22(2):557-66. doi: 10.3748/wjg.v22.i2.557. World J Gastroenterol. 2016. PMID: 26811607 Free PMC article. Review.
-
Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: role of circ_0000977/miR-153 axis.RNA Biol. 2019 Nov;16(11):1592-1603. doi: 10.1080/15476286.2019.1649585. Epub 2019 Aug 12. RNA Biol. 2019. PMID: 31402756 Free PMC article.
-
MicroRNA-200c Promotes Suppressive Potential of Myeloid-Derived Suppressor Cells by Modulating PTEN and FOG2 Expression.PLoS One. 2015 Aug 18;10(8):e0135867. doi: 10.1371/journal.pone.0135867. eCollection 2015. PLoS One. 2015. PMID: 26285119 Free PMC article.
Cited by
-
miR-155 and miR-21 as Diagnostic and Therapeutic Biomarkers for Ulcerative Colitis: There Is Still a Long Way to Go.Biomedicines. 2024 Jun 13;12(6):1315. doi: 10.3390/biomedicines12061315. Biomedicines. 2024. PMID: 38927522 Free PMC article.
-
The Oncogenic Burden of Obesity: Mechanistic Links Between Adiposity and Gastrointestinal Cancers-A Comprehensive Narrative Review.Biomedicines. 2025 Jun 26;13(7):1571. doi: 10.3390/biomedicines13071571. Biomedicines. 2025. PMID: 40722646 Free PMC article. Review.
-
Influence of the gut microbiota on immune cell interactions and cancer treatment.J Transl Med. 2024 Oct 15;22(1):939. doi: 10.1186/s12967-024-05709-3. J Transl Med. 2024. PMID: 39407240 Free PMC article. Review.
-
Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies.Cancers (Basel). 2025 Apr 23;17(9):1408. doi: 10.3390/cancers17091408. Cancers (Basel). 2025. PMID: 40361336 Free PMC article. Review.
-
Decoding the pancreatic cancer microenvironment: The multifaceted regulation of microRNAs.Clin Transl Med. 2025 Jul;15(7):e70354. doi: 10.1002/ctm2.70354. Clin Transl Med. 2025. PMID: 40579785 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

