Humoral Response to mRNA-1273 SARS-CoV-2 Vaccine in Peritoneal Dialysis Patients: Is Boostering After Six Months Adequate?
- PMID: 35814775
- PMCID: PMC9263093
- DOI: 10.3389/fmed.2022.905798
Humoral Response to mRNA-1273 SARS-CoV-2 Vaccine in Peritoneal Dialysis Patients: Is Boostering After Six Months Adequate?
Abstract
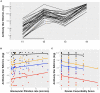
In dialysis patients the humoral response to anti-SARS-CoV-2 vaccines is attenuated and rapidly declines over time. However, data on the persistence of the immune response in peritoneal dialysis (PD) patients are scarce, particularly after a third (booster) dose with mRNA-1273 vaccine. In this prospective cohort study, we report anti-SARS-CoV-2 antibody levels in PD patients before and after the third dose of mRNA-1273 vaccine. Six months after the second dose, anti-SARS-CoV-2 antibodies were detected in all patients (n = 34). However, within this time period antibodies substantially declined in 31 of 34 patients (4.5-fold, median = 192 BAU/mL, p = 1.27 × 10-9) and increased in three patients. In accordance with government regulations, a third dose of mRNA-1273 vaccine (50 μg) was given to 27 PD patients 6 months after the second dose which induced a significant increase of anti-SARS-CoV-2 antibody titers (58.6-fold, median = 19405 BAU/mL, p = 1.24 × 10-29). A mixed model analysis showed that a lower Davies Comorbidity Score and a higher GFR were associated with higher antibody titers (p = 0.03 and p = 0.02). The most common adverse events after the third dose were pain at the injection site (77.8%) and fatigue (51.9%). No hospitalizations were reported. In conclusion, 6 months after the second dose of mRNA-1273 vaccine, anti-SARS-CoV-2 antibodies substantially decreased in PD patients, whereas a well-tolerated third dose induced a robust humoral response. Our data suggest that the administration of a booster dose within a shorter interval than 6 months should be considered in PD patients in order to maintain high anti-SARS-CoV-2 antibody levels and assure protection from severe COVID-19 disease.
Keywords: COVID-19; anti-SARS-CoV-2 antibodies; booster; mRNA-1273 vaccine; peritoneal dialysis; spikevax.
Copyright © 2022 Beilhack, Monteforte, Frommlet, Reindl-Schwaighofer, Strassl and Vychytil.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Long-Term Dynamic Humoral Response to SARS-CoV-2 mRNA Vaccines in Patients on Peritoneal Dialysis.Vaccines (Basel). 2022 Oct 18;10(10):1738. doi: 10.3390/vaccines10101738. Vaccines (Basel). 2022. PMID: 36298603 Free PMC article.
-
Durable Anti-SARS-CoV-2 Antibody Response after mRNA-1273 Booster in Peritoneal Dialysis Patients during the Omicron Wave.Vaccines (Basel). 2023 Jun 19;11(6):1121. doi: 10.3390/vaccines11061121. Vaccines (Basel). 2023. PMID: 37376510 Free PMC article.
-
Antibody Response and Safety After mRNA-1273 SARS-CoV-2 Vaccination in Peritoneal Dialysis Patients - the Vienna Cohort.Front Immunol. 2021 Dec 2;12:780594. doi: 10.3389/fimmu.2021.780594. eCollection 2021. Front Immunol. 2021. PMID: 34925359 Free PMC article.
-
SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis.Am J Kidney Dis. 2022 Feb;79(2):185-192.e1. doi: 10.1053/j.ajkd.2021.08.005. Epub 2021 Sep 8. Am J Kidney Dis. 2022. PMID: 34508833 Free PMC article.
-
Waning of SARS-CoV-2 Vaccine-Induced Immune Response over 6 Months in Peritoneal Dialysis Patients and the Role of a Booster Dose in Maintaining Seropositivity.Nephron. 2022;146(6):559-563. doi: 10.1159/000524658. Epub 2022 May 20. Nephron. 2022. PMID: 35598596 Free PMC article.
Cited by
-
Long-Term Dynamic Humoral Response to SARS-CoV-2 mRNA Vaccines in Patients on Peritoneal Dialysis.Vaccines (Basel). 2022 Oct 18;10(10):1738. doi: 10.3390/vaccines10101738. Vaccines (Basel). 2022. PMID: 36298603 Free PMC article.
-
Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2022 Dec 2;10(12):2070. doi: 10.3390/vaccines10122070. Vaccines (Basel). 2022. PMID: 36560480 Free PMC article. Review.
-
Efficacy and safety of booster vaccination against SARS-CoV-2 in dialysis and renal transplant patients: systematic review and meta-analysis.Int Urol Nephrol. 2023 Apr;55(4):791-802. doi: 10.1007/s11255-023-03471-x. Epub 2023 Feb 1. Int Urol Nephrol. 2023. PMID: 36723829 Free PMC article.
-
COVID-19 Vaccination Among Patients Receiving Maintenance Renal Replacement Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future.J Infect Dis. 2023 Aug 4;228(Suppl 1):S46-S54. doi: 10.1093/infdis/jiad162. J Infect Dis. 2023. PMID: 37539761 Free PMC article.
-
Durable Anti-SARS-CoV-2 Antibody Response after mRNA-1273 Booster in Peritoneal Dialysis Patients during the Omicron Wave.Vaccines (Basel). 2023 Jun 19;11(6):1121. doi: 10.3390/vaccines11061121. Vaccines (Basel). 2023. PMID: 37376510 Free PMC article.
References
-
- Davidovic T, Schimpf J, Abbassi-Nik A, Stockinger R, Sprenger-Mahr H, Lhotta K, et al. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. (2021) 100:1334–5. 10.1016/j.kint.2021.10.006 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

