Clinical Outcomes of Patients With Unresectable Primary Liver Cancer Treated With Yttrium-90 Radioembolization With an Escalated Dose
- PMID: 35814852
- PMCID: PMC9260102
- DOI: 10.1016/j.adro.2022.100948
Clinical Outcomes of Patients With Unresectable Primary Liver Cancer Treated With Yttrium-90 Radioembolization With an Escalated Dose
Abstract
Purpose: Yttrium-90 (90Y) radioembolization with an escalated dose has been shown to improve clinical outcomes compared with standard dose radioembolization, but there are few data on the local control of primary liver tumors. We reported the clinical outcomes of patients with unresectable primary liver tumors treated with 90Y radioembolization with an escalated dose.
Methods and materials: Clinical data of patients with unresectable hepatocellular carcinoma (HCC), cholangiocarcinoma (CC), and biphenotypic tumors (cHCC-CC) treated with radioembolization with an escalated dose (≥150 Gy) between 2013 and 2020 with >3 months follow-up were retrospectively reviewed. The primary endpoint was freedom from local progression. Clinical response was defined by Modified Response Evaluation Criteria in Solid Tumours and toxic effects were assessed using Common Terminology Criteria for Adverse Events version 5.0.
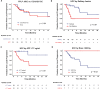
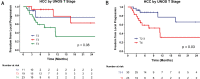
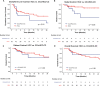
Results: Fifty-three patients with HCC and 15 patients with CC/cHCC-CC were analyzed. The median dose delivered was 205 Gy (interquartile range, 183-253 Gy) and 198 Gy (interquartile range, 154-234 Gy) for patients with HCC and CC/cHCC-CC, respectively. The 1-year freedom from local progression rate was 54% (95% confidence interval [CI], 38%-78%) for patients with HCC and 66% (95% CI, 42%-100%) for patients with CC/cHCC-CC. For patients with HCC, United Network for Organ Sharing nodal stage 1 (P = .01), nonsolitary tumors (P = .02), pretreatment α-fetoprotein of >7.7 ng/mL (P = .006), and ≤268 Gy dose delivered (P = .003) were predictors for local progression on multivariate Cox analysis. No patients with HCC who received a dose >268 Gy had a local tumor progression. The 1-year overall survival for patients with HCC was 74% (95% CI, 61%-89%). After radioembolization, 5 (7%) patients had grade 3 ascites, and 4 (6%) patients had grade 3/4 hyperbilirubinemia.
Conclusions: Treatment of unresectable primary liver tumors with 90Y radioembolization with an escalated dose was safe and well tolerated. Delivery of >268 Gy may improve local tumor control of HCC. Determination of the maximum tolerated dose needs to be performed in the context of future prospective dose-escalation trials to further evaluate the safety and efficacy of such an approach.
© 2022 The Authors.
Figures
Similar articles
-
Outcomes of Yttrium-90 Radioembolization for Unresectable Combined Biphenotypic Hepatocellular-Cholangiocarcinoma.J Vasc Interv Radiol. 2020 May;31(5):701-709. doi: 10.1016/j.jvir.2019.09.028. Epub 2020 Feb 29. J Vasc Interv Radiol. 2020. PMID: 32127318
-
Yttrium-90 Radioembolization for Unresectable Combined Hepatocellular-Cholangiocarcinoma.Cardiovasc Intervent Radiol. 2017 Sep;40(9):1383-1391. doi: 10.1007/s00270-017-1648-7. Epub 2017 Apr 21. Cardiovasc Intervent Radiol. 2017. PMID: 28432387
-
Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma.J Gastrointest Oncol. 2015 Oct;6(5):469-78. doi: 10.3978/j.issn.2078-6891.2015.056. J Gastrointest Oncol. 2015. PMID: 26487939 Free PMC article.
-
Radioembolization of Intrahepatic Cholangiocarcinoma: Patient Selection, Outcomes, and Competing Therapies.Semin Intervent Radiol. 2021 Oct;38(4):438-444. doi: 10.1055/s-0041-1735526. Epub 2021 Oct 7. Semin Intervent Radiol. 2021. PMID: 34629711 Free PMC article. Review.
-
Contemporary Algorithm for the Management of Hepatocellular Carcinoma in 2021: The Northwestern Approach.Semin Intervent Radiol. 2021 Oct;38(4):432-437. doi: 10.1055/s-0041-1735528. Epub 2021 Oct 7. Semin Intervent Radiol. 2021. PMID: 34629710 Free PMC article. Review.
Cited by
-
Yttrium-90 Transarterial Radioembolization of Primary Lung Cancer Metastases to the Liver.J Vasc Interv Radiol. 2024 Feb;35(2):214-225.e2. doi: 10.1016/j.jvir.2023.10.025. Epub 2023 Nov 3. J Vasc Interv Radiol. 2024. PMID: 37923172 Free PMC article.
-
Holmium-166 Transarterial Radioembolization for the Treatment of Intrahepatic Cholangiocarcinoma: A Case Series.Cancers (Basel). 2023 Sep 29;15(19):4791. doi: 10.3390/cancers15194791. Cancers (Basel). 2023. PMID: 37835485 Free PMC article.
-
Systemic Immunological Changes After Yttrium-90 Radioembolization: A Pilot Prospective Observational Study-Clinical Insights.Cardiovasc Intervent Radiol. 2024 Nov;47(11):1461-1470. doi: 10.1007/s00270-024-03870-2. Epub 2024 Oct 15. Cardiovasc Intervent Radiol. 2024. PMID: 39406871
-
Arterial hypoperfusion as a negative predictive marker for primary hepatic malignancies treated with Y-90 glass microsphere transarterial radioembolization.Front Oncol. 2024 Aug 7;14:1433480. doi: 10.3389/fonc.2024.1433480. eCollection 2024. Front Oncol. 2024. PMID: 39169947 Free PMC article.
-
Stereotactic body radiation therapy (SBRT) following Yttrium-90 (90Y) selective internal radiation therapy (SIRT): a feasibility planning study using90Y delivered dose.Phys Med Biol. 2023 Mar 10;68(6):065003. doi: 10.1088/1361-6560/acbbb5. Phys Med Biol. 2023. PMID: 36780696 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. - PubMed
-
- Fortner JG, Maclean BJ, Kim DK, et al. The seventies evolution in liver surgery for cancer. Cancer. 1981;47:2162–2166. - PubMed
-
- Heckman JT, Devera MB, Marsh JW, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Annal Surg Oncol. 2008;15:3169–3177. - PubMed
-
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92–99. - PubMed
LinkOut - more resources
Full Text Sources

