Efficacy and safety of corticosteroid regimens for the treatment of hospitalized COVID-19 patients: a meta-analysis
- PMID: 35814934
- PMCID: PMC9249165
- DOI: 10.2217/fvl-2021-0244
Efficacy and safety of corticosteroid regimens for the treatment of hospitalized COVID-19 patients: a meta-analysis
Abstract
Aim: To evaluate the efficacy and safety of corticosteroids for treating hospitalized COVID-19 patients.
Materials & methods: Efficacy outcomes included time to negative SARS-CoV-2 tests, length of stay, duration and incidence of intensive unit care stay, incidence of mortality and duration and incidence of mechanical ventilation. Safety outcomes included the incidence of adverse events and severe adverse events, incidence of hyperglycemia and incidence of nosocomial infections.
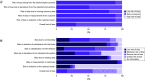
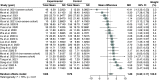
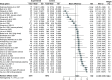
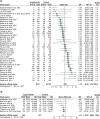
Results: Ninety-five randomized controlled trials (RCTs) and observational studies (n = 42,205) were included. Corticosteroids were associated with increased length of stay (based on RCT only), increased time to negative tests, decreased length of mechanical ventilation and increased odds of hyperglycemia.
Conclusion: Corticosteroids should be considered in patients requiring mechanical ventilation, and glycemic monitoring may be needed when administering corticosteroids.
Keywords: COVID-19; SARS-CoV-2; corticosteroids; length of stay; mechanical ventilation; mortality.
© 2022 Future Medicine Ltd.
Figures



Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Evaluating the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 8;21(1):499. doi: 10.1186/s13063-020-04422-y. Trials. 2020. PMID: 32513308 Free PMC article.
-
Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes.Crit Care. 2020 Dec 14;24(1):696. doi: 10.1186/s13054-020-03400-9. Crit Care. 2020. PMID: 33317589 Free PMC article.
-
Efficacy of chloroquine and hydroxychloroquine for the treatment of hospitalized COVID-19 patients: a meta-analysis.Future Virol. 2021 Nov:10.2217/fvl-2021-0119. doi: 10.2217/fvl-2021-0119. Epub 2021 Dec 3. Future Virol. 2021. PMID: 34887938 Free PMC article. Review.
-
Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis.Leukemia. 2020 Jun;34(6):1503-1511. doi: 10.1038/s41375-020-0848-3. Epub 2020 May 5. Leukemia. 2020. PMID: 32372026 Free PMC article.
Cited by
-
Efficacy and Safety of Hydrocortisone, Ascorbic Acid, and Thiamine Combination Therapy for the Management of Sepsis and Septic Shock: A Systematic Review and Meta-Analysis of Randomised Controlled Trials.Int Arch Allergy Immunol. 2024;185(10):997-1018. doi: 10.1159/000538959. Epub 2024 Jun 13. Int Arch Allergy Immunol. 2024. PMID: 38870923 Free PMC article.
-
Persistent symptoms and risk factors predicting prolonged time to symptom-free after SARS‑CoV‑2 infection: an analysis of the baseline examination of the German COVIDOM/NAPKON-POP cohort.Infection. 2023 Dec;51(6):1679-1694. doi: 10.1007/s15010-023-02043-6. Epub 2023 May 25. Infection. 2023. PMID: 37231313 Free PMC article.
-
Drugs for COVID-19: An Update.Molecules. 2022 Dec 5;27(23):8562. doi: 10.3390/molecules27238562. Molecules. 2022. PMID: 36500655 Free PMC article. Review.
-
The Quest for Secondary Pharmaceuticals: Drug Repurposing/Chiral-Switches Combination Strategy.ACS Pharmacol Transl Sci. 2023 Jan 17;6(2):201-219. doi: 10.1021/acsptsci.2c00151. eCollection 2023 Feb 10. ACS Pharmacol Transl Sci. 2023. PMID: 36798472 Free PMC article. Review.
-
Effects of Drugs Formerly Proposed for COVID-19 Treatment on Connexin43 Hemichannels.Int J Mol Sci. 2022 Apr 30;23(9):5018. doi: 10.3390/ijms23095018. Int J Mol Sci. 2022. PMID: 35563409 Free PMC article.
References
-
- Mahase E. COVID-19: WHO declares pandemic because of ‘alarming levels’ of spread, severity, and inaction. BMJ 368, m1036 (2020). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
