Transcriptomic Analysis Reveals Key Candidate Genes Related to Seed Abortion in Chinese Jujube (Ziziphus jujuba Mill)
- PMID: 35814940
- PMCID: PMC9199538
- DOI: 10.2174/1389202922666211110100017
Transcriptomic Analysis Reveals Key Candidate Genes Related to Seed Abortion in Chinese Jujube (Ziziphus jujuba Mill)
Abstract
Background: Seed abortion is a common phenomenon in Chinese jujube that seriously hinders the process of cross-breeding. However, the molecular mechanisms of seed abortion remain unclear in jujube.
Methods: Here, we performed transcriptome sequencing using eight flower and fruit tissues at different developmental stages in Ziziphus jujuba Mill. 'Zhongqiusucui' to identify key genes related to seed abortion. Histological analysis revealed a critical developmental process of embryo abortion after fertilization.
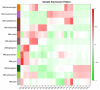
Results: Comparisons of gene expression revealed a total of 14,012 differentially expressed genes. Functional enrichment analyses of differentially expressed genes between various sample types uncovered several important biological processes, such as embryo development, cellular metabolism, and stress response, that were potentially involved in the regulation of seed abortion. Furthermore, gene co-expression network analysis revealed a suite of potential key genes related to ovule and seed development. We focused on three types of candidate genes, agamous subfamily genes, plant ATP-binding cassette subfamily G transporters, and metacaspase enzymes, and showed that the expression profiles of some members were associated with embryo abortion.
Conclusion: This work generates a comprehensive gene expression data source for unraveling the molecular mechanisms of seed abortion and aids future cross-breeding efforts in jujube.
Keywords: Ziziphus jujuba Mill; breeding; candidate genes; embryo abortion; seed abortion; transcriptome.
© 2022 Bentham Science Publishers.
Figures




Similar articles
-
Integrated microRNA and transcriptome profiling reveals the regulatory network of embryo abortion in jujube.Tree Physiol. 2023 Jan 5;43(1):142-153. doi: 10.1093/treephys/tpac098. Tree Physiol. 2023. PMID: 35972818 Free PMC article.
-
De novo assembly and characterization of the fruit transcriptome of Chinese jujube (Ziziphus jujuba Mill.) Using 454 pyrosequencing and the development of novel tri-nucleotide SSR markers.PLoS One. 2014 Sep 3;9(9):e106438. doi: 10.1371/journal.pone.0106438. eCollection 2014. PLoS One. 2014. PMID: 25184704 Free PMC article.
-
Genome-Wide Identification of WRKY Transcription Factors in Chinese jujube (Ziziphus jujuba Mill.) and Their Involvement in Fruit Developing, Ripening, and Abiotic Stress.Genes (Basel). 2019 May 10;10(5):360. doi: 10.3390/genes10050360. Genes (Basel). 2019. PMID: 31083435 Free PMC article.
-
Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review.Food Chem. 2017 Jul 15;227:349-357. doi: 10.1016/j.foodchem.2017.01.074. Epub 2017 Jan 17. Food Chem. 2017. PMID: 28274443 Review.
-
Wild Jujube (Ziziphus jujuba var. spinosa): A Review of Its Phytonutrients, Health Benefits, Metabolism, and Applications.J Agric Food Chem. 2022 Jul 6;70(26):7871-7886. doi: 10.1021/acs.jafc.2c01905. Epub 2022 Jun 22. J Agric Food Chem. 2022. PMID: 35731918 Review.
References
-
- Li J.W., Fan L.P., Ding S.D., Ding X.L. Nutritional composition of five cultivars of Chinese jujube. Food Chem. 2007;103(2):454–460. doi: 10.1016/j.foodchem.2006.08.016. - DOI
-
- Liu M.J. The present status, problems and countermeasures of Chinese jujube production. Rev. China Agric. Sci. Technol. 2000;2:76–80.
-
- Liu M.J., Wang J.R., Liu P., Zhao J., Zhao Z.H., Dai L., Li X.S., Liu Z.G. Historical achievements and frontier advances in the production and research of Chinese jujube (Ziziphus jujuba) in China. Yuan Yi Xue Bao. 2015;42(9):1683–1698.
-
- Qu Z.Z., Wang Y.H. China fruit’s monograph-Chinese jujube volume. Vol. 56. For. Publ. House: Beijing, China; 1993. p. 229.
LinkOut - more resources
Full Text Sources
