β-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Weighing the Challenges and Opportunities to Assess Clinical Value
- PMID: 35815181
- PMCID: PMC9259115
- DOI: 10.1097/CCE.0000000000000726
β-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Weighing the Challenges and Opportunities to Assess Clinical Value
Abstract
Objective: β-lactams are the cornerstone of empiric and targeted antibiotic therapy for critically ill patients. Recently, there have been calls to use β-lactam therapeutic drug monitoring (TDM) within 24-48 hours after the initiation of therapy in critically ill patients. In this article, we review the dynamic physiology of critically ill patients, β-lactam dose response in critically ill patients, the impact of pathogen minimum inhibitory concentration (MIC) on β-lactam TDM, and pharmacokinetics in critically ill patients. Additionally, we highlight available clinical data to better inform β-lactam TDM for critically ill patients.
Data sources: We retrospectively analyzed patients admitted for sepsis or septic shock at a single academic medical center who were treated with β-lactam antibiotics.
Study selection: Indexed studies in PubMed in English language were selected for review on topics relative to critical care physiology, β-lactams, pharmacokinetics/pharmacodynamics, TDM, and antibiotic susceptibility.
Data extraction: We reviewed potentially related studies on β-lactams and TDM and summarized their design, patients, and results. This is a synthetic, nonsystematic, review.
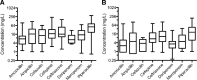
Data synthesis: In the retrospective analysis of patients treated with β-lactam antibiotics, approximately one-third of patients received less than 48 hours of β-lactam therapy. Of those who continued beyond 48 hours, only 13.7% had patient-specific factors (augmented renal clearance, fluid overload, morbid obesity, and/or surgical drain), suggesting a potential benefit of β-lactam TDM.
Conclusions: These data indicate that a strategy of comprehensive β-lactam TDM for critically ill patients is unwarranted as it has not been shown yet to improve patient-oriented outcomes. This review demonstrates that β-lactam TDM in the ICU, while laudable, layers ambiguous β-lactam exposure thresholds upon uncertain/unknown MIC data within a dynamic, unpredictable patient population for whom TDM results will not be available fast enough to significantly affect care. Judicious, targeted TDM for those with risk factors for β-lactam over- or underexposure is a better approach but requires further study. Clinically, choosing the correct antibiotic and dosing β-lactams aggressively, which have a wide therapeutic index, to overcome critical illness factors appears to give critically ill patients the best likelihood of survival.
Keywords: beta-lactam antibiotics; critically ill; intensive care unit; pharmacodynamics; pharmacokinetics; therapeutic drug monitoring.
Copyright © 2022 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Dr. Schulz reports grant funding from Merck. Dr. Micek reports being a coinvestigator on a grant from Merck. Dr. Kollef reports salary support from the Barnes-Jewish Hospital Foundation. Dr. Rose reports research funding from Merck and Paratek and personal consulting fees from Paratek and Visante. Dr. Dilworth has disclosed that he does not have any potential conflicts of interest.
Figures

Similar articles
-
Impact of β-lactam antibiotic therapeutic drug monitoring on dose adjustments in critically ill patients undergoing continuous renal replacement therapy.Int J Antimicrob Agents. 2017 May;49(5):589-594. doi: 10.1016/j.ijantimicag.2017.01.009. Epub 2017 Mar 21. Int J Antimicrob Agents. 2017. PMID: 28341612
-
The effect of therapeutic drug monitoring of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients: the DOLPHIN trial protocol of a multi-centre randomised controlled trial.BMC Infect Dis. 2020 Jan 17;20(1):57. doi: 10.1186/s12879-020-4781-x. BMC Infect Dis. 2020. PMID: 31952493 Free PMC article. Clinical Trial.
-
Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients.Crit Care. 2018 Sep 24;22(1):233. doi: 10.1186/s13054-018-2155-1. Crit Care. 2018. PMID: 30244674 Free PMC article. Review.
-
Provider perspectives on beta-lactam therapeutic drug monitoring programs in the critically ill: a protocol for a multicenter mixed-methods study.Implement Sci Commun. 2021 Mar 24;2(1):34. doi: 10.1186/s43058-021-00134-9. Implement Sci Commun. 2021. PMID: 33762025 Free PMC article.
-
Towards precision medicine: Therapeutic drug monitoring-guided dosing of vancomycin and β-lactam antibiotics to maximize effectiveness and minimize toxicity.Am J Health Syst Pharm. 2020 Jul 7;77(14):1104-1112. doi: 10.1093/ajhp/zxaa128. Am J Health Syst Pharm. 2020. PMID: 32537644 Review.
Cited by
-
Therapeutic Drug Monitoring of Antimicrobials in Critically Ill Obese Patients.Antibiotics (Basel). 2023 Jun 24;12(7):1099. doi: 10.3390/antibiotics12071099. Antibiotics (Basel). 2023. PMID: 37508195 Free PMC article.
-
Outcomes of Intravenous Push versus Intermittent Infusion Administration of Cefepime in Critically Ill Patients.Antibiotics (Basel). 2023 Jun 1;12(6):996. doi: 10.3390/antibiotics12060996. Antibiotics (Basel). 2023. PMID: 37370315 Free PMC article.
-
The clinical application of beta-lactam antibiotic therapeutic drug monitoring in the critical care setting.J Antimicrob Chemother. 2023 Oct 3;78(10):2395-2405. doi: 10.1093/jac/dkad223. J Antimicrob Chemother. 2023. PMID: 37466209 Free PMC article. Review.
-
Treatment of critically ill patients with cefiderocol for infections caused by multidrug-resistant pathogens: review of the evidence.Ann Intensive Care. 2023 Jun 15;13(1):52. doi: 10.1186/s13613-023-01146-5. Ann Intensive Care. 2023. PMID: 37322293 Free PMC article. Review.
-
Development and Validation of a Capillary Zone Electrophoresis-Tandem Mass Spectrometry Method for Simultaneous Quantification of Eight β-Lactam Antibiotics and Two β-Lactamase Inhibitors in Plasma Samples.Pharmaceuticals (Basel). 2024 Apr 19;17(4):526. doi: 10.3390/ph17040526. Pharmaceuticals (Basel). 2024. PMID: 38675486 Free PMC article.
References
-
- Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. ; Infection Section of European Society of Intensive Care Medicine (ESICM); Pharmacokinetic/pharmacodynamic and Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases (ESCMID); Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT); Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC): Antimicrobial therapeutic drug monitoring in critically ill adult patients: A position paper. Intensive Care Med 2020; 46:1127–1153 - PMC - PubMed
-
- Craig WA: Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26:1–10 - PubMed
-
- Dhaese S, Heffernan A, Liu D, et al. : Prolonged versus intermittent infusion of β-lactam antibiotics: A systematic review and meta-regression of bacterial killing in preclinical infection models. Clin Pharmacokinet 2020; 59:1237–1250 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

