Rhodopsins: An Excitingly Versatile Protein Species for Research, Development and Creative Engineering
- PMID: 35815212
- PMCID: PMC9257189
- DOI: 10.3389/fchem.2022.879609
Rhodopsins: An Excitingly Versatile Protein Species for Research, Development and Creative Engineering
Abstract
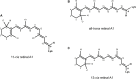
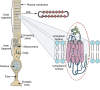
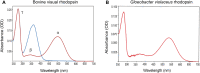
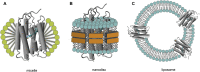
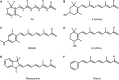
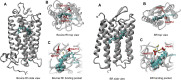
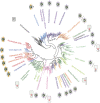
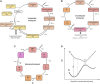
The first member and eponym of the rhodopsin family was identified in the 1930s as the visual pigment of the rod photoreceptor cell in the animal retina. It was found to be a membrane protein, owing its photosensitivity to the presence of a covalently bound chromophoric group. This group, derived from vitamin A, was appropriately dubbed retinal. In the 1970s a microbial counterpart of this species was discovered in an archaeon, being a membrane protein also harbouring retinal as a chromophore, and named bacteriorhodopsin. Since their discovery a photogenic panorama unfolded, where up to date new members and subspecies with a variety of light-driven functionality have been added to this family. The animal branch, meanwhile categorized as type-2 rhodopsins, turned out to form a large subclass in the superfamily of G protein-coupled receptors and are essential to multiple elements of light-dependent animal sensory physiology. The microbial branch, the type-1 rhodopsins, largely function as light-driven ion pumps or channels, but also contain sensory-active and enzyme-sustaining subspecies. In this review we will follow the development of this exciting membrane protein panorama in a representative number of highlights and will present a prospect of their extraordinary future potential.
Keywords: eukaryotic; ion pumps; membrane protein; microbial; optogenetics; photoreceptor; retinal protein; visual pigments.
Copyright © 2022 de Grip and Ganapathy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abdulaev N. G., Artamonov I. D., Bogachuk A. S., Feigina M. Y., Kostina M. B., Kudelin A. B., et al. (1982). Structure of Light-Activated Proteins - Visual Rhodopsin. Biochem. Int. 5, 693–703.
-
- Acharya A. R., Vandekerckhove B., Larsen L. E., Delbeke J., Wadman W. J., Vonck K., et al. (2021). In Vivo blue Light Illumination for Optogenetic Inhibition: Effect on Local Temperature and Excitability of the Rat hippocampus. J. Neural Eng. 18, 066038–066031. 10.1088/1741-2552/ac3ef4 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

