Synthesis and Biological Evaluation of 5-Fluoro-2-Oxindole Derivatives as Potential α-Glucosidase Inhibitors
- PMID: 35815213
- PMCID: PMC9261963
- DOI: 10.3389/fchem.2022.928295
Synthesis and Biological Evaluation of 5-Fluoro-2-Oxindole Derivatives as Potential α-Glucosidase Inhibitors
Abstract
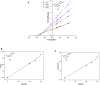
α-Glucosidase inhibitors are known to prevent the digestion of carbohydrates and reduce the impact of carbohydrates on blood glucose. To develop novel α-glucosidase inhibitors, a series of 5-fluoro-2-oxindole derivatives (3a ∼ 3v) were synthesized, and their α-glucosidase inhibitory activities were investigated. Biological assessment results showed that most synthesized compounds presented potential inhibition on α-glucosidase. Among them, compounds 3d, 3f, and 3i exhibited much better inhibitory activity with IC50 values of 49.89 ± 1.16 μM, 35.83 ± 0.98 μM, and 56.87 ± 0.42 μM, respectively, which were about 10 ∼ 15 folds higher than acarbose (IC50 = 569.43 ± 43.72 μM). A kinetic mechanism study revealed that compounds 3d, 3f, and 3i inhibited the α-glucosidase in a reversible and mixed manner. Molecular docking was carried out to simulate the affinity between the compound and α-glucosidase.
Keywords: docking; inhibition; kinetics; oxindole; α-glucosidase.
Copyright © 2022 Lin, Liang, Ye, Xiao, Lu, Li, Li, Zhang, Xiong, Feng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Al-Salahi R., Ahmad R., Anouar E., Iwana Nor Azman N. I., Marzouk M., Abuelizz H. A. (2018). 3-Benzyl(phenethyl)-2-thioxobenzo[g]quinazolines as a New Class of Potent α-glucosidase Inhibitors: Synthesis and Molecular Docking Study. Future Med. Chem. 10, 1889–1905. 10.4155/fmc-2018-0141 - DOI - PubMed
-
- Chaudhry F., Naureen S., Ashraf M., Al-Rashida M., Jahan B., Munawar M. A., et al. (2019). Imidazole-pyrazole Hybrids: Synthesis, Characterization and In-Vitro Bioevaluation against α-glucosidase Enzyme with Molecular Docking Studies. Bioorg. Chem. 82, 267–273. 10.1016/j.bioorg.2018.10.047 - DOI - PubMed
LinkOut - more resources
Full Text Sources

