Renoprotective and Cardioprotective Potential of Moricandia sinaica (Boiss.) against Carbon Tetrachloride-Induced Toxicity in Rats
- PMID: 35815261
- PMCID: PMC9259224
- DOI: 10.1155/2022/8545695
Renoprotective and Cardioprotective Potential of Moricandia sinaica (Boiss.) against Carbon Tetrachloride-Induced Toxicity in Rats
Abstract
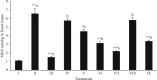
The goal of the current study was to assess the nephroprotective and cardioprotective potential of Moricandia sinaica methanol extract (MOR-1), as well as its butanol (MOR-2) and aqueous (MOR-3) fractions against carbon tetrachloride (CCl4)-induced nephro and cardio-toxicity. Cardiac function was assessed using the biochemical parameters lactate dehydrogenase (LDH) and creatinine kinase (CK). Renal function was examined using the biochemical parameters creatinine and uric acid. The levels of nonprotein sulfhydryls (NPSH) and malondialdehyde (MDA) were used as markers of oxidative strain. A dose of 100 and 200 mg/kg of butanol fraction given prior to CCl4 treatment significantly (p < 0.05 - 0.001) protected against elevated LDH and CK levels. Similarly, treatment with silymarin (10 mg/kg) and butanol fraction (100 and 200 mg/kg) significantly (p < 0.05 - 0.001) boosted total protein levels compared to CCl4 treatment alone. The silymarin (10 mg/kg) and butanol fraction (100 and 200 mg/kg) also provided a significant (p < 0.05 - 0.001) protective effect for MDA levels. Methanol extract (MOR-1) and butanol (MOR-2) showed significant results and were recommended for further pharmacological and screening for active constituents.
Copyright © 2022 Abdelaaty A. Shahat et al.
Conflict of interest statement
The authors have declared that they have no conflicts of interest.
Figures








References
-
- Mahesh B., Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World Journal of Agricultural Sciences . 2008;4:839–843.
-
- Shahed-Al-Mahmud M., Lina S. M. M. Evaluation of sedative and anxiolytic activities of methanol extract of leaves of persicaria hydropiper in mice. Clinical Phytoscience . 2017;3:p. 20. doi: 10.1186/s40816-017-0056-5. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

