A versatile design platform for glycoengineering therapeutic antibodies
- PMID: 35815437
- PMCID: PMC9272841
- DOI: 10.1080/19420862.2022.2095704
A versatile design platform for glycoengineering therapeutic antibodies
Abstract
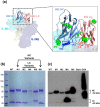
Manipulation of glycosylation patterns, i.e., glycoengineering, is incorporated in the therapeutic antibody development workflow to ensure clinical safety, and this approach has also been used to modulate the biological activities, functions, or pharmacological properties of antibody drugs. Whereas most existing glycoengineering strategies focus on the canonical glycans found in the constant domain of immunoglobulin G (IgG) antibodies, we report a new strategy to leverage the untapped potential of atypical glycosylation patterns in the variable domains, which naturally occur in 15% to 25% of IgG antibodies. Glycosylation sites were added to the antigen-binding regions of two functionally divergent, interleukin-2-binding monoclonal antibodies. We used computational tools to rationally install various N-glycosylation consensus sequences into the antibody variable domains, creating "glycovariants" of these molecules. Strikingly, almost all the glycovariants were successfully glycosylated at their newly installed N-glycan sites, without reduction of the antibody's native function. Importantly, certain glycovariants exhibited modified activities compared to the parent antibody, showing the potential of our glycoengineering strategy to modulate biological function of antibodies involved in multi-component receptor systems. Finally, when coupled with a high-flux sialic acid precursor, a glycovariant with two installed glycosylation sites demonstrated superior in vivo half-life. Collectively, these findings validate a versatile glycoengineering strategy that introduces atypical glycosylation into therapeutic antibodies in order to improve their efficacy and, in certain instances, modulate their activity early in the drug development process.
Keywords: ManNAc analog; Protein glycoengineering; glycosylation; immunotherapy; interleukin-2; metabolic glycoengineering; sialylation; therapeutics.
Conflict of interest statement
The authors have filed intellectual property claims covering the technologies described herein.
Figures




References
-
- van de Bovenkamp Fs, Derksen NIL, Ooijevaar-de Heer P, van Schie Ka, Heer P O-D, Berkowska MA, van der Schoot Ce, IJspeert H, van der Burg M, Gils A, et al. Adaptive antibody diversification through N -linked glycosylation of the immunoglobulin variable region. Proc Natl Acad Sci. 2018;115(8):1901–06. doi:10.1073/pnas.1711720115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
