Nogo-A reduces ceramide de novo biosynthesis to protect from heart failure
- PMID: 35815623
- PMCID: PMC10226746
- DOI: 10.1093/cvr/cvac108
Nogo-A reduces ceramide de novo biosynthesis to protect from heart failure
Abstract
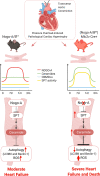
Aims: Growing evidence correlate the accrual of the sphingolipid ceramide in plasma and cardiac tissue with heart failure (HF). Regulation of sphingolipid metabolism in the heart and the pathological impact of its derangement remain poorly understood. Recently, we discovered that Nogo-B, a membrane protein of endoplasmic reticulum, abundant in the vascular wall, down-regulates the sphingolipid de novo biosynthesis via serine palmitoyltransferase (SPT), first and rate liming enzyme, to impact vascular functions and blood pressure. Nogo-A, a splice isoform of Nogo, is transiently expressed in cardiomyocyte (CM) following pressure overload. Cardiac Nogo is up-regulated in dilated and ischaemic cardiomyopathies in animals and humans. However, its biological function in the heart remains unknown.
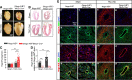
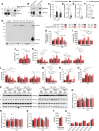
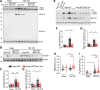
Methods and results: We discovered that Nogo-A is a negative regulator of SPT activity and refrains ceramide de novo biosynthesis in CM exposed to haemodynamic stress, hence limiting ceramide accrual. At 7 days following transverse aortic constriction (TAC), SPT activity was significantly up-regulated in CM lacking Nogo-A and correlated with ceramide accrual, particularly very long-chain ceramides, which are the most abundant in CM, resulting in the suppression of 'beneficial' autophagy. At 3 months post-TAC, mice lacking Nogo-A in CM showed worse pathological cardiac hypertrophy and dysfunction, with ca. 50% mortality rate.
Conclusion: Mechanistically, Nogo-A refrains ceramides from accrual, therefore preserves the 'beneficial' autophagy, mitochondrial function, and metabolic gene expression, limiting the progression to HF under sustained stress.
Keywords: Autophagy; Ceramide; Heart failure; Mitochondrial function; Sphingolipid metabolism.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: None declared.
Figures


References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics 2019. Update: a report from the American Heart Association. Circulation 2019;139:e56–e528. - PubMed
-
- Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 2002;277:25847–25850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

