Analysis of 52 240 source plasma donors of convalescent COVID-19 plasma: Sex, ethnicity, and age association with initial antibody levels and rate of dissipation
- PMID: 35815776
- PMCID: PMC9350246
- DOI: 10.1002/jca.21998
Analysis of 52 240 source plasma donors of convalescent COVID-19 plasma: Sex, ethnicity, and age association with initial antibody levels and rate of dissipation
Abstract
Background: COVID-19 convalescent plasma (CCP) was approved under emergency authorization to treat critically ill patients with COVID-19 in the United States in 2020. We explored the demographics of donors contributing plasma for a hyperimmune, plasma-derived therapy to evaluate factors that may be associated with anti-SARS-CoV-2 antibody response variability and, subsequently, antibody titers.
Study design: An electronic search of CCP donors was performed across 282 US plasma donation centers. Donations were screened for nucleocapsid protein-binding-IgG using the Abbott SARS-CoV-2 IgG assay.
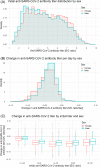
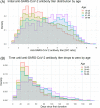
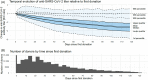
Results: Overall, 52 240 donors donated 418 046 units of CCP. Donors were of various ethnicities: 43% Caucasian, 34% Hispanic, 17% African American, 2% Native American, 1% Asian, and 3% other. Females had higher initial mean anti-SARS-CoV-2 antibody titers but an overall faster rate of decline (P < .0001). Initial antibody titers increased with age: individuals aged 55 to 66 years had elevated anti-SARS-CoV-2 titers for longer periods compared with other ages (P = .0004). African American donors had the lowest initial antibody titers but a slower rate of decline (P < .0001), while Caucasian (P = .0088) and Hispanic (P = .0193) groups had the fastest rates of decline. Most donor antibody levels decreased below the inclusion criteria (≥1.50) within 30 to 100 days of first donation, but donation frequency did not appear to be associated with rate of decline.
Conclusion: Several factors may be associated with anti-SARS-CoV-2 antibody response including donor age and sex. Evaluating these factors during development of future hyperimmune globulin products may help generation of therapies with optimal efficacy.
Keywords: SARS-CoV-2; convalescent COVID-19 plasma; hyperimmune globulins; plasma donor demographics.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
All of the authors are employees of CSL Plasma or CSL Behring.
Figures
Similar articles
-
Longitudinal analysis of SARS-CoV-2 antibodies in 8000 U.S. first-time convalescent plasma donations.Transfusion. 2021 Apr;61(4):1141-1147. doi: 10.1111/trf.16291. Epub 2021 Feb 22. Transfusion. 2021. PMID: 33615484 Free PMC article.
-
Blood group O convalescent plasma donations have significantly lower levels of SARS-CoV-2 IgG antibodies compared to blood group A donations.Transfusion. 2021 Aug;61(8):2245-2249. doi: 10.1111/trf.16524. Epub 2021 May 27. Transfusion. 2021. PMID: 34036595 Free PMC article.
-
SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors.J Infect Dis. 2020 Nov 13;222(12):1974-1984. doi: 10.1093/infdis/jiaa581. J Infect Dis. 2020. PMID: 32910175 Free PMC article.
-
How should we use convalescent plasma therapies for the management of COVID-19?Blood. 2021 Mar 25;137(12):1573-1581. doi: 10.1182/blood.2020008903. Blood. 2021. PMID: 33202419 Free PMC article. Review.
-
Regulatory consideration on preparation and clinical use of COVID-19 convalescent plasma.Transfus Apher Sci. 2020 Oct;59(5):102846. doi: 10.1016/j.transci.2020.102846. Epub 2020 Jun 4. Transfus Apher Sci. 2020. PMID: 32593519 Free PMC article. Review.
Cited by
-
A Spike-destructing human antibody effectively neutralizes Omicron-included SARS-CoV-2 variants with therapeutic efficacy.PLoS Pathog. 2023 Jan 27;19(1):e1011085. doi: 10.1371/journal.ppat.1011085. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36706160 Free PMC article.
-
A novel highly specific biotinylated MAC-ELISA for detection of anti-SARS-CoV-2 nucleocapsid antigen IgM antibodies during the acute phase of COVID-19.Braz J Microbiol. 2023 Dec;54(4):2893-2901. doi: 10.1007/s42770-023-01160-6. Epub 2023 Nov 6. Braz J Microbiol. 2023. PMID: 37930615 Free PMC article.
-
Predictors of high SARS-CoV-2 immunoglobulin G titers in COVID-19 convalescent whole-blood donors: a cross-sectional study in China.Front Immunol. 2023 Jun 14;14:1191479. doi: 10.3389/fimmu.2023.1191479. eCollection 2023. Front Immunol. 2023. PMID: 37388736 Free PMC article.
References
-
- Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80‐90. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

