When and how do we stop antifungal treatment for an invasive mould infection in allogeneic haematopoietic cell transplant recipients?
- PMID: 35815918
- PMCID: PMC9796773
- DOI: 10.1111/myc.13496
When and how do we stop antifungal treatment for an invasive mould infection in allogeneic haematopoietic cell transplant recipients?
Abstract
Background: Limited data exist to describe end-of-treatment (EOT) parameters of antifungal therapy for invasive mould infections (IMI).
Methods: In a 10-year cohort of consecutive adult allogeneic haematopoietic cell transplant recipients with proven/probable IMI, we describe treatment duration and patient profile at EOT.
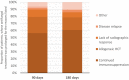
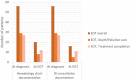
Results: There were 61 patients with 66 proven/probable IMI identified: 47/66 (71%) invasive aspergillosis (IA), 11/66 (17%) mucormycosis, and 8/66 (12%) other-IMI. Excluding 5 (8%) patients lost to follow-up, treatment was prematurely discontinued due to death or palliative care in 29/56 (51.8%) patients. Antifungal treatment was completed in 27 (48.2%) patients, for a median duration of 280 days (IQR: 110, 809): 258 (IQR: 110, 1905) and 307.5 (99, 809) days in IA and non-IA IMI, respectively. Treatment was continued after 90 and 180 days in 43/56 (76.8%) and 30/56 (53.6%) patients, respectively. At EOT, most patients were not neutropenic (ANC: 2.12 G/L, IQR: 0.04, 5.3), with CD4+ counts at 99 cells/μl (IQR: 0, 759) and immunoglobulins at 5.6 g/L (IQR: 2.3, 10.6). Most patients (16/27, 59.3%) were not receiving steroids at EOT, while 14/27 (53.9%) were on another type of immunosuppression. Amongst 15 patients with imaging at EOT, 12 (80%) had complete/partial radiologic response. Any chart documentation or an infectious disease consultation on treatment discontinuation was observed in 12/56 (21%) and 11/56 (20%) patients, respectively.
Conclusions: Long treatment courses are observed in patients with IMI, due to prolonged immunosuppression. Although immune reconstitution and radiological response were frequently observed at EOT, consistent documentation of treatment discontinuation based on well-defined parameters is lacking.
Keywords: allogeneic haematopoietic cell transplant recipients; antifungal treatment discontinuation; antifungal treatment stop; invasive mould infections.
© 2022 The Authors. Mycoses published by Wiley-VCH GmbH.
Conflict of interest statement
R.S.R., S.M‐L., F.G., A‐C.M. and C.v.D.: No conflicts of interest. Y.C. has received consulting fees from MSD, Novartis, Incyte, BMS, Pfizer, Abbvie, Roche, Jazz, Gilead, Amgen, Astra‐Zeneka Servier and travel support from MSD, Roche, Gilead, Amgen, Incite, Abbvie, Janssen, Astra‐Zeneka, Jazz. D.N. has received research support from MSD and Pfizer and consulting fees from Roche Diagnostics, MSD, Pfizer, Basilea and Gilead.
Figures

References
-
- Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408‐415. - PubMed
-
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81‐89. - PubMed
-
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by aspergillus and other filamentous fungi (SECURE): a phase 3, randomised‐controlled, non‐inferiority trial. Lancet. 2016;387(10020):760‐769. - PubMed
-
- Maertens JA, Rahav G, Lee DG, et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non‐inferiority trial. Lancet. 2021;397(10273):499‐509. - PubMed
-
- Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high‐loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007;44(10):1289‐1297. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

