Polythioesters Prepared by Ring-Opening Polymerization of Cyclic Thioesters and Related Monomers
- PMID: 35816010
- PMCID: PMC9543045
- DOI: 10.1002/asia.202200641
Polythioesters Prepared by Ring-Opening Polymerization of Cyclic Thioesters and Related Monomers
Abstract
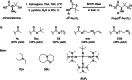
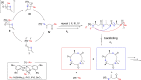
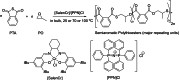
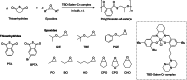
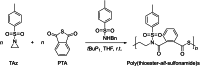
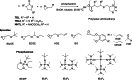
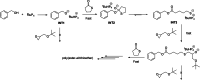
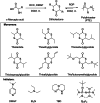
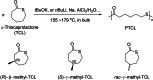
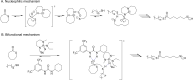
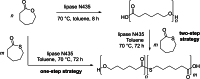
Polyhydroxyalkanoates (PHAs) are biodegradable and biocompatible polyesters with a wide range of applications; in particular, they currently stand as promising alternatives to conventional polyolefin-based "plastics". The introduction of sulfur atoms within the PHAs backbone can endow the resulting polythioesters (PTEs) with differentiated, sometimes enhanced thermal, optical and mechanical properties, thereby widening their versatility and use. Hence, PTEs have been gaining increasing attention over the past half-decade. This review highlights recent advances towards the synthesis of well-defined PTEs by ring-opening polymerization (ROP) of cyclic thioesters - namely thiolactones - as well as of S-carboxyanhydrides and thionolactones; it also covers the ring-opening copolymerization (ROCOP) of cyclic thioanhydrides or thiolactones with epoxides or episulfides. Most of the ROP reactions described are of anionic type, mediated by inorganic, organic or organometallic initiators/catalysts, along with a few enzymatic reactions as well. Emphasis is placed on the reactivity of the thio monomers, in relation to their ring-size ranging from 4- to 5-, 6- and 7-membered cycles, the nature of the catalyst/initiating systems implemented and their efficiency in terms of activity and control over the PTE molar mass, dispersity, topology, and microstructure.
Keywords: Degradability; Polythioester; Recyclability; Ring-Opening Copolymerization (ROCOP); Ring-Opening Polymerization (ROP); Thioester; Thiolactone.
© 2022 The Authors. Chemistry - An Asian Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

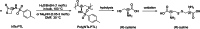
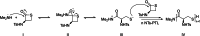










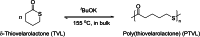
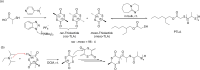


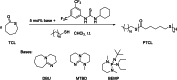



References
-
- None
-
- Xu G., Wang Q., Green Chem. 2022, 24, 2321–2346;
-
- Haider T. P., Volker C., Kramm J., Landfester K., Wurm F. R., Angew. Chem. Int. Ed. 2019, 58, 50–62; - PubMed
- Angew. Chem. 2019, 131, 50–63;
-
- Coates G., Getzler Y. D. Y. L., Nat. Rev. Mater. 2020, 5, 501–516;
-
- Jehanno C., Alty J. W., Roosen M., De Meester S., Dove A. P., Chen E. Y.-X., Leibfarth F. A., Sardon H., Nature 2022, 603, 803–814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

