Long-term Immune Response to SARS-CoV-2 Infection Among Children and Adults After Mild Infection
- PMID: 35816313
- PMCID: PMC9280400
- DOI: 10.1001/jamanetworkopen.2022.21616
Long-term Immune Response to SARS-CoV-2 Infection Among Children and Adults After Mild Infection
Abstract
Importance: Understanding the long-term immune response against SARS-CoV-2 infection in children is crucial to optimize vaccination strategies. Although it is known that SARS-CoV-2 antibodies may persist in adults 12 months after infection, data are limited in the pediatric population.
Objective: To examine long-term anti-SARS-CoV-2 spike receptor-binding domain (S-RBD) IgG kinetics in children after SARS-CoV-2 infection.
Design, setting, and participants: In this single-center, prospective cohort study, patients were enrolled consecutively from April 1, 2020, to August 31, 2021, at the COVID-19 Family Cluster Follow-up Clinic, Department of Women's and Children's Health, University Hospital of Padua. A cohort of 252 COVID-19 family clusters underwent serologic follow-up at 1 to 4, 5 to 10, and more than 10 months after infection with quantification of anti-S-RBD IgG by chemiluminescent immunoassay.
Exposures: SARS-CoV-2 infection.
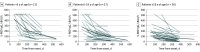
Results: Among 902 study participants, 697 had confirmed SARS-CoV-2 infection, including 351 children or older siblings (mean [SD] age, 8.6 [5.1] years) and 346 parents (mean [SD] age, 42.5 [7.1] years). Among 697 cases, 674 (96.7%) were asymptomatic or mild. Children had significantly higher S-RBD IgG titers than older patients across all follow-up time points, with an overall median S-RBD IgG titer in patients younger than 3 years 5-fold higher than adults (304.8 [IQR, 139.0-516.6] kBAU/L vs 55.6 [24.2-136.0] kBAU/L, P < .001). Longitudinal analysis of 56 study participants sampled at least twice during follow-up demonstrated the persistence of antibodies up to 10 months from infection in all age classes, despite a progressive decline over time.
Conclusions and relevance: In this cohort study of Italian children and adults following SARS-CoV-2 infection different kinetics of SARS-CoV-2 antibodies were found across several age classes of individuals with asymptomatic or mild COVID-19, which could help in optimizing COVID-19 vaccination strategies and prevention policies. This work provides further evidence of sustained immune response in children up to 1 year after primary SARS-CoV-2 infection.
Conflict of interest statement
Figures


References
-
- Li L, Zhang W, Hu Y, et al. . Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. [published correction appears in JAMA. 2020 Aug 4;324(5):519]. JAMA. 2020;324(5):460-470. doi:10.1001/jama.2020.10044 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

