G protein-coupled receptor signaling: transducers and effectors
- PMID: 35816644
- PMCID: PMC9448338
- DOI: 10.1152/ajpcell.00210.2022
G protein-coupled receptor signaling: transducers and effectors
Abstract
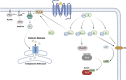
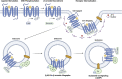
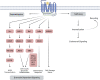
G protein-coupled receptors (GPCRs) are of considerable interest due to their importance in a wide range of physiological functions and in a large number of Food and Drug Administration (FDA)-approved drugs as therapeutic entities. With continued study of their function and mechanism of action, there is a greater understanding of how effector molecules interact with a receptor to initiate downstream effector signaling. This review aims to explore the signaling pathways, dynamic structures, and physiological relevance in the cardiovascular system of the three most important GPCR signaling effectors: heterotrimeric G proteins, GPCR kinases (GRKs), and β-arrestins. We will first summarize their prominent roles in GPCR pharmacology before transitioning into less well-explored areas. As new technologies are developed and applied to studying GPCR structure and their downstream effectors, there is increasing appreciation for the elegance of the regulatory mechanisms that mediate intracellular signaling and function.
Keywords: G protein; G protein-coupled receptor; GPCR kinase; effector; transducer; β-arrestin.
Conflict of interest statement
H. A. Rockman is a scientific cofounder of Trevena Inc., a company that is developing new GPCR ligands. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
This article is part of the special collection “Advances in GPCRs: Structure, Mechanisms, Disease, and Pharmacology.” Wei Kong, MD, PhD, and Jinpeng Sun, PhD, served as Guest Editors of this collection.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

