Endothelin-1 as a novel target for the prevention of metabolic dysfunction with intermittent hypoxia in male participants
- PMID: 35816718
- PMCID: PMC9423726
- DOI: 10.1152/ajpregu.00301.2021
Endothelin-1 as a novel target for the prevention of metabolic dysfunction with intermittent hypoxia in male participants
Abstract
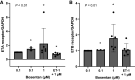
We examined the effect of intermittent hypoxia (IH, a hallmark feature of sleep apnea) on adipose tissue lipolysis and the role of endothelin-1 (ET-1) in this response. We hypothesized that IH can increase ET-1 secretion and plasma free fatty acid (FFA) concentrations. We further hypothesized that inhibition of ET-1 receptor activation with bosentan could prevent any IH-mediated increase in FFA. To test this hypothesis, 16 healthy male participants (32 ± 5 yr, 26 ± 2 kg/m2) were exposed to 30 min of IH in the absence (control) and presence of bosentan (62.5 mg oral twice daily for 3 days prior). Arterial blood samples for ET-1, epinephrine, and FFA concentrations, as well as abdominal subcutaneous adipose tissue biopsies (to assess transcription of cellular receptors/proteins involved in lipolysis), were collected. Additional proof-of-concept studies were conducted in vitro using primary differentiated human white preadipocytes (HWPs). We show that IH increased circulating ET-1, epinephrine, and FFA (P < 0.05). Bosentan treatment reduced plasma epinephrine concentrations and blunted IH-mediated increases in FFA (P < 0.01). In adipose tissue, bosentan had no effect on cellular receptors and proteins involved in lipolysis (P > 0.05). ET-1 treatment did not directly induce lipolysis in differentiated HWP. In conclusion, IH increases plasma ET-1 and FFA concentrations. Inhibition of ET-1 receptors with bosentan attenuates the FFA increase in response to IH. Based on a lack of a direct effect of ET-1 in HWP, we speculate the effect of bosentan on circulating FFA in vivo may be secondary to its ability to reduce sympathoadrenal tone.
Keywords: adipose tissue; bosentan; epinephrine; fatty acids; intermittent hypoxia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






Similar articles
-
Endothelin-1 receptor blockade does not alter the sympathetic and hemodynamic response to acute intermittent hypoxia in men.J Appl Physiol (1985). 2022 Oct 1;133(4):867-875. doi: 10.1152/japplphysiol.00837.2021. Epub 2022 Aug 11. J Appl Physiol (1985). 2022. PMID: 35952348 Free PMC article.
-
Endothelin regulates intermittent hypoxia-induced lipolytic remodelling of adipose tissue and phosphorylation of hormone-sensitive lipase.J Physiol. 2016 Mar 15;594(6):1727-40. doi: 10.1113/JP271321. Epub 2016 Jan 20. J Physiol. 2016. PMID: 26663321 Free PMC article.
-
NFATc3 contributes to intermittent hypoxia-induced arterial remodeling in mice.Am J Physiol Heart Circ Physiol. 2010 Aug;299(2):H356-63. doi: 10.1152/ajpheart.00341.2010. Epub 2010 May 21. Am J Physiol Heart Circ Physiol. 2010. PMID: 20495147 Free PMC article.
-
Endothelin in the pulmonary circulation with special reference to hypoxic pulmonary vasoconstriction.Scand Cardiovasc J Suppl. 1997;46:1-40. Scand Cardiovasc J Suppl. 1997. PMID: 9265559 Review.
-
Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea.Best Pract Res Clin Endocrinol Metab. 2010 Oct;24(5):843-51. doi: 10.1016/j.beem.2010.08.011. Best Pract Res Clin Endocrinol Metab. 2010. PMID: 21112030 Free PMC article. Review.
Cited by
-
Endothelin-1 receptor blockade does not alter the sympathetic and hemodynamic response to acute intermittent hypoxia in men.J Appl Physiol (1985). 2022 Oct 1;133(4):867-875. doi: 10.1152/japplphysiol.00837.2021. Epub 2022 Aug 11. J Appl Physiol (1985). 2022. PMID: 35952348 Free PMC article.
References
-
- Barceló A, Piérola J, de la Peña M, Esquinas C, Fuster A, Sanchez-de-la-Torre M, Carrera M, Alonso-Fernandez A, Ladaria A, Bosch M, Barbé F. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J 37: 1418–1423, 2011. doi:10.1183/09031936.00050410. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

