Seeking heterocyclic scaffolds as antivirals against dengue virus
- PMID: 35816877
- PMCID: PMC9250831
- DOI: 10.1016/j.ejmech.2022.114576
Seeking heterocyclic scaffolds as antivirals against dengue virus
Abstract
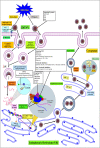
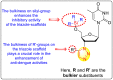
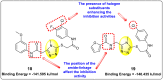
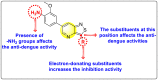
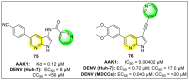
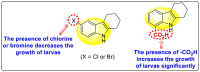
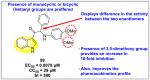
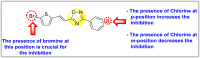
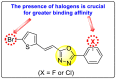
Dengue is one of the most typical viral infection categorized in the Neglected Tropical Diseases (NTDs). It is transmitted via the female Aedes aegypti mosquito to humans and majorly puts risk to the lives of more than half of the world. Recent advancements in medicinal chemistry have led to the design and development of numerous potential heterocyclic scaffolds as antiviral drug candidates for the inhibition of the dengue virus (DENV). Thus, in this review, we have discussed the significance of inhibitory and antiviral activities of nitrogen, oxygen, and mixed (nitrogen-sulfur and nitrogen-oxygen) heterocyclic scaffolds that are published in the last seven years (2016-2022). Furthermore, we have also discussed the probable mechanisms of action and the diverse structure-activity relationships (SARs) of the heterocyclic scaffolds. In addition, this review has elaborately outlined the mechanism of viral infection and the life cycle of DENV in the host cells. The wide set of heterocycles and their SARs will aid in the development of pharmaceuticals that will allow the researchers to synthesize the promising anti-dengue drug candidate in the future.
Keywords: Antiviral medication; Dengue virus; Heterocyclic scaffolds; Neglected tropical diseases; Structure-activity relationship; Surface activity.
Copyright © 2022 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures











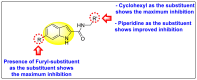


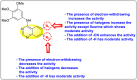
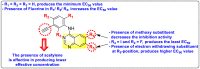
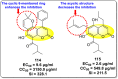


References
-
- World Health Organization Dengue and severe dengue. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue n.d.
-
- Guzman M.G., Halstead S.B., Artsob H., Buchy P., Farrar J., Gubler D.J., Hunsperger E., Kroeger A., Margolis H.S., Martí-nez E., Nathan M.B., Pelegrino J.L., Simmons C., Yoksan S., Peeling R.W. Dengue: a continuing global threat. Nat. Rev. Microbiol. 2010;8 doi: 10.1038/nrmicro2460. S7–S16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

