A transdiagnostic review of safety, efficacy, and parameter space in accelerated transcranial magnetic stimulation
- PMID: 35816982
- PMCID: PMC10029148
- DOI: 10.1016/j.jpsychires.2022.06.038
A transdiagnostic review of safety, efficacy, and parameter space in accelerated transcranial magnetic stimulation
Abstract
Background: Accelerated transcranial magnetic stimulation (aTMS) is an emerging delivery schedule of repetitive TMS (rTMS). TMS is "accelerated" by applying two or more stimulation sessions within a day. This three-part review comprehensively reports the safety/tolerability, efficacy, and stimulation parameters affecting response across disorders.
Methods: We used the PubMed database to identify studies administering aTMS, which we defined as applying at least two rTMS sessions within one day.
Results: Our targeted literature search identified 85 aTMS studies across 18 diagnostic and healthy control groups published from July 2001 to June 2022. Excluding overlapping populations, 63 studies delivered 43,873 aTMS sessions using low frequency, high frequency, and theta burst stimulation in 1543 participants. Regarding safety, aTMS studies had similar seizure and side effect incidence rates to those reported for once daily rTMS. One seizure was reported from aTMS (0.0023% of aTMS sessions, compared with 0.0075% in once daily rTMS). The most common side effects were acute headache (28.4%), fatigue (8.6%), and scalp discomfort (8.3%), with all others under 5%. We evaluated aTMS efficacy in 23 depression studies (the condition with the most studies), finding an average response rate of 42.4% and remission rate of 28.4% (range = 0-90.5% for both). Regarding parameters, aTMS studies ranged from 2 to 10 sessions per day over 2-30 treatment days, 10-640 min between sessions, and a total of 9-104 total accelerated TMS sessions per participant (including tapering sessions). Qualitatively, response rate tends to be higher with an increasing number of sessions per day, total sessions, and total pulses.
Discussion: The literature to date suggests that aTMS is safe and well-tolerated across conditions. Taken together, these early studies suggest potential effectiveness even in highly treatment refractory conditions with the added potential to reduce patient burden while also expediting response time. Future studies are warranted to systematically investigate how key aTMS parameters affect treatment outcome and durability.
Keywords: Accelerated TMS (aTMS); High dose rTMS; Intensive rTMS; Theta burst stimulation; Transcranial magnetic stimulation; Transdiagnostic review.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


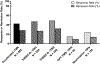
Comment in
-
Letter to the editor: Safety of "accelerated" rTMS protocols with twice-daily sessions in patients with schizophrenia - A comment on Caulfield et al.J Psychiatr Res. 2022 Dec;156:754-757. doi: 10.1016/j.jpsychires.2022.08.025. Epub 2022 Sep 2. J Psychiatr Res. 2022. PMID: 36088124 No abstract available.
-
Reply to "Letter to the editor: Safety of "accelerated" rTMS protocols with twice-daily sessions in patients with schizophrenia - A comment on Caulfield et al.".J Psychiatr Res. 2022 Dec;156:758-760. doi: 10.1016/j.jpsychires.2022.08.021. Epub 2022 Sep 2. J Psychiatr Res. 2022. PMID: 36123170 No abstract available.
References
-
- Baeken C, De Witte S, Tandt H, Vervaet J, Lemmens GM, 2020a. Accelerated theta burst stimulation in a case of therapy-resistant depression developed after left anterior temporal lobectomy. Brain Stimul.: Basic, Transl. Clin. Res. Neuromodul 13 (6), 1519–1520. - PubMed
-
- Baeken C, Duprat R, Wu G-R, De Raedt R, van Heeringen K, 2017a. Subgenual anterior cingulate–medial orbitofrontal functional connectivity in medication- resistant major depression: a neurobiological marker for accelerated intermittent theta burst stimulation treatment? Biol. Psychiatr.: Cognit. Neurosci. Neuroimag 2 (7), 556–565. - PubMed
-
- Baeken C, Lefaucheur J-P, Van Schuerbeek P, 2017b. The impact of accelerated high frequency rTMS on brain neurochemicals in treatment-resistant depression: insights from 1H MR spectroscopy. Clin. Neurophysiol 128 (9), 1664–1672. - PubMed
-
- Baeken C, Marinazzo D, Everaert H, Wu G-R, Van Hove C, Audenaert K, Goethals I, De Vos F, Peremans K, De Raedt R, 2015. The impact of accelerated HF-rTMS on the subgenual anterior cingulate cortex in refractory unipolar major depression: insights from 18FDG PET brain imaging. Brain Stimul. 8 (4), 808–815. - PubMed
-
- Baeken C, Marinazzo D, Wu G-R, Van Schuerbeek P, De Mey J, Marchetti I, Vanderhasselt M-A, Remue J, Luypaert R, De Raedt R, 2014. Accelerated HF- rTMS in treatment-resistant unipolar depression: insights from subgenual anterior cingulate functional connectivity. World J. Biol. Psychiatr 15 (4), 286–297. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

