Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial
- PMID: 35817072
- PMCID: PMC9270059
- DOI: 10.1016/S2213-2600(22)00215-6
Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial
Erratum in
-
Correction to Lancet Respir Med 2022; 10: 972-84.Lancet Respir Med. 2022 Dec;10(12):e116. doi: 10.1016/S2213-2600(22)00441-6. Epub 2022 Nov 7. Lancet Respir Med. 2022. PMID: 36356600 Free PMC article. No abstract available.
Abstract
Background: Tixagevimab-cilgavimab is a neutralising monoclonal antibody combination hypothesised to improve outcomes for patients hospitalised with COVID-19. We aimed to compare tixagevimab-cilgavimab versus placebo, in patients receiving remdesivir and other standard care.
Methods: In a randomised, double-blind, phase 3, placebo-controlled trial, adults with symptoms for up to 12 days and hospitalised for COVID-19 at 81 sites in the USA, Europe, Uganda, and Singapore were randomly assigned in a 1:1 ratio to receive intravenous tixagevimab 300 mg-cilgavimab 300 mg or placebo, in addition to remdesivir and other standard care. Patients were excluded if they had acute organ failure including receipt of invasive mechanical ventilation, extracorporeal membrane oxygenation, vasopressor therapy, mechanical circulatory support, or new renal replacement therapy. The study drug was prepared by an unmasked pharmacist; study participants, site study staff, investigators, and clinical providers were masked to study assignment. The primary outcome was time to sustained recovery up to day 90, defined as 14 consecutive days at home after hospital discharge, with co-primary analyses for the full cohort and for participants who were neutralising antibody-negative at baseline. Efficacy and safety analyses were done in the modified intention-to-treat population, defined as participants who received a complete or partial infusion of tixagevimab-cilgavimab or placebo. This study is registered with ClinicalTrials.gov, NCT04501978 and the participant follow-up is ongoing.
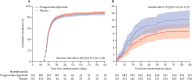
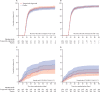
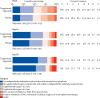
Findings: From Feb 10 to Sept 30, 2021, 1455 patients were randomly assigned and 1417 in the primary modified intention-to-treat population were infused with tixagevimab-cilgavimab (n=710) or placebo (n=707). The estimated cumulative incidence of sustained recovery was 89% for tixagevimab-cilgavimab and 86% for placebo group participants at day 90 in the full cohort (recovery rate ratio [RRR] 1·08 [95% CI 0·97-1·20]; p=0·21). Results were similar in the seronegative subgroup (RRR 1·14 [0·97-1·34]; p=0·13). Mortality was lower in the tixagevimab-cilgavimab group (61 [9%]) versus placebo group (86 [12%]; hazard ratio [HR] 0·70 [95% CI 0·50-0·97]; p=0·032). The composite safety outcome occurred in 178 (25%) tixagevimab-cilgavimab and 212 (30%) placebo group participants (HR 0·83 [0·68-1·01]; p=0·059). Serious adverse events occurred in 34 (5%) participants in the tixagevimab-cilgavimab group and 38 (5%) in the placebo group.
Interpretation: Among patients hospitalised with COVID-19 receiving remdesivir and other standard care, tixagevimab-cilgavimab did not improve the primary outcome of time to sustained recovery but was safe and mortality was lower.
Funding: US National Institutes of Health (NIH) and Operation Warp Speed.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests TLH reports consulting fees from Lysovant, royalties for UpToDate topic authorship, and participation on a Staphylococcus Aureus Network Adaptive Platform Trial Data and Safety Monitoring Board (DSMB), outside of the submitted work. AAG reports grants from US National Institutes of Health (NIH) during the conduct of the study, grants from US Centers for Disease Control (CDC), US Department of Defense (DOD), AbbVie, and Faron Pharmaceuticals, and participation on a NIH DSMB, outside of the submitted work. RP reports grants from Gilead, ViiV, and MSD and consulting fees from Gilead, ViiV, MSD, Theratechnologies, and Eli Lilly, outside of the submitted work. TAM reports grants from National Institute of Allergies and Infectious Diseases (NIAID), NIH, and Leidos, outside of the submitted work. GG reports partial salary support from NIH through the University of Minnesota during the conduct of the study. US reports grants from ViiV, Cytodyn, NIAID, and Cardiothoracic Surgical Trial Network, consulting fees from ViiV and Shionogi, and payment for educational events from Paratek and Shionogi, outside of the submitted work. RLG reports contracts from Regeneron, consulting fees from Gilead and GSK Pharmaceuticals, participation on advisory boards for Eli Lilly, Gilead, GSK, Johnson and Johnson, Roche–Genentech, and Kinevant Sciences, de minimis stock in AbCellera, and receipt of medication from Gilead as an in-kind gift to facilitate the conduct of an academic-sponsored clinical trial, outside of the submitted work. MKJ reports grants from Gilead, participation on an advisory board for Gilead, and a Board of Director position with the HIV Medication Association, outside of the submitted work. LM reports payment for educational events from Merck, travel support from Merck and Gilead, and participation on an advisory board for Merck, outside of the submitted work. EM reports grants from NIAID and NIH, outside of the submitted work. KK reports grants and contracts with NIH, Regeneron, Abbott, Pfizer, Romark, and Raisonance, consulting fees from Regeneron, travel support from Sanford Guide and Burroughs Wellcome Fund, a patent pending for 3D swabs, and leadership positions at Sanford Guide and Burroughs Wellcome Fund, outside of the submitted work. PEM reports grants from NHLBI and NIH during the conduct of the study, grants and contracts from NHLBI and NIH, and consulting fees from Dompe, Medtronic, and Boerhinger Ingelheim, outside of the submitted work. BT reports grants from VA Cooperative Studies Program Coordinating Center during the conduct of the study and grants and consulting fees from Genentech, outside of the study. KUK reports grants from NIH during the conduct of the study. MAM reports grants from NIH, DOD, the California Institute of Regenerative Medicine, Roche-Genentech, and Quantum Therapeutics and consulting fees from Novartis, Johnson and Johnson, Citius Pharmaceuticals, Pilant Therapeutics, and Gilead, outside of the submitted work. GP reports consulting fees from Menarini, Pfizer, MSD, and Gilead, payment for education events from Pfizer, MSD, AstraZeneca, and Gilead, and participation on advisory boards for Pfizer, Gilead, and MSD, outside of the submitted work. KNS reports consulting fees from Roche, Bristol Myers Squibb (BMS), Amgen, and MSD, outside of the submitted work. SRB reports consulting fees from Gilead, MSD, and GSK, payment for educational events from Gilead and MSD, and participation on advisory boards for Gilead, MSD, Pfizer, Roche, and ViiV, outside of the submitted work. JDC reports grants from NIH, outside of the submitted work. PC reports grants from NIH during the conduct of the study and grants from Eli Lilly, Regeneron, and Gilead, consulting fees from Eli Lilly, Regeneron, and Gilead, and payment for education events from Frontier Collaborative, Physician Education Resource, Rockpointe, CME Outfitter, outside of the submitted work. DJD reports grants from NIH during the conduct of the study. DCF reports grants from NIH during the conduct of the study, grants from NIH and CDC, consulting fees from Cytovale, and participation on a DSMB for Medpace, outside of the submitted work. HFG reports grants from the Swiss National Science Foundation, Swiss HIV Cohort Study, Gilead, the Yvonne Jacob Foundation, and NIH and participation on advisory boards for Merck, Gilead, ViiV, Janssen, and Novartis, outside of the submitted work. RDH reports grants from NHLBI, Airway Therapeutics, Incyte Corporation, and Kiniksa Pharmaceuticals and participation on a NIH DSMB outside of the submitted work. AK reports grants from United Therapeutics, Johnson & Johnson, Eli Lilly, AstraZeneca, and 4DMedical, outside of the submitted work. JSO reports grants from NIH during the conduct of the study. JSS reports grants from NIH during the conduct of the study. BEY reports consulting fees from Gilead and Novacyte and payment for educational events from AstraZeneca, Gilead, Sanofi, and Roche, outside of the submitted work. ANP reports grants from Bill and Melinda Gates Foundation (BMGF), UK Research and Innovation (UKRI), the Wellcome Trust, and the National Institute for Health and Care Research (NIHR) and consulting fees from BMGF, outside of the submitted work. DDM reports support from DNRF126 during the conduct of the study. MLP reports grants from Boehringer-Ingelheim, payment for authorship from Genentech and France Foundation, and travel support from Eastern Pulmonary Conference, outside of the submitted work. DS reports salary support from NIH through Axle Informatics during the conduct of the study and a leadership position for EigenMed, outside of the submitted work. VN reports grants from NIH during the conduct of the study. SLP reports grants from the University of Minnesota during the conduct of the study and grants from Gilead, ViiV, Janssen, the European and Developing Countries Clinical Trials Partnership, the Medical Reserve Corps (MRC), and NIHR, and participation on a NIHR DSMB, outside of the submitted work. GT reports grants from University College London, outside of the submitted work. SMB reports grants from NIH during the conduct of the study and grants from NIH and participation on a DSMB for Hamilton, outside of the submitted work. WHS reports grants from NIH and NHLBI during the conduct of the study. BG reports grants from NIH during the conduct of the study. SS reports grants from NIH, outside of the conduct of the study. CSR reports grants from NIH during the conduct of the study. PG reports being an employee of AstraZeneca, being a full member of Academia de Medicina, Rio de Janeiro, Brazil, and stock ownership at Takeda. MTE reports stock ownership and being an employee at AstraZeneca. AT reports and stock ownership and being an employee at AstraZeneca. AGB reports grants from University of Minnesota, MRC, and UKRI during the conduct of the study. VJD reports grants from NIAID–NIH and a contract with the University of Minnesota, outside of the conduct of the study. GM reports grants from Gilead, AbbVie, and Viiv, payment for a speaking engagement from Janssen, participation on advisory boards for AstraZeneca and Gilead, and a leadership role on the Australian National COVID-19 taskforce treatments committee, outside of the conduct of the study. BTT reports grants from NHLBI and consulting fees from Bayer, Novartis, and Thetis, outside of the conduct of the study. JDN reports grants from NIH and NIAID during the conduct of the study. All other members of the writing committee declare no competing interests.
Figures

Comment in
-
Monoclonals for patients hospitalised with COVID-19.Lancet Respir Med. 2022 Oct;10(10):928-930. doi: 10.1016/S2213-2600(22)00222-3. Epub 2022 Jul 8. Lancet Respir Med. 2022. PMID: 35817073 Free PMC article. No abstract available.
-
Cardiac and vascular serious adverse events following tixagevimab-cilgavimab - Author's reply.Lancet Respir Med. 2023 Jan;11(1):e7-e8. doi: 10.1016/S2213-2600(22)00450-7. Epub 2022 Dec 12. Lancet Respir Med. 2023. PMID: 36521509 Free PMC article. No abstract available.
-
Cardiac and vascular serious adverse events following tixagevimab-cilgavimab.Lancet Respir Med. 2023 Jan;11(1):e5-e6. doi: 10.1016/S2213-2600(22)00452-0. Epub 2022 Dec 12. Lancet Respir Med. 2023. PMID: 36521510 No abstract available.
References
-
- Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med. 2021;385:1941–1950. - PubMed
-
- US Food and Drug Administration . US Food and Drug Administration; Silver Spring, MD: 2022. Letter of authorization for emergency use of EVUSHELD (tixagevimab and cilgavimab)https://www.fda.gov/media/154704/download
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

